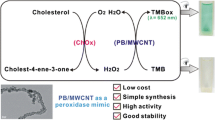
Abstract
Fe3O4-brominated graphene (Fe3O4-GBR) nanocomposites were synthesized via an in situ method using the precursors FeSO4.7H2O and GBR in different (1:1, 1:2, 2:1, 1:5, 1:10, 1:20, and 5:1) weight ratios at pH 11.5. The Fe3O4-GBR (1:5) nanocomposite in combination with H2O2 and 3,3′,5,5′-tetramethylbenzidine (TMB) showed swift and superior intrinsic peroxidase mimetic enzyme activity compared with the other Fe3O4-GBR composites, GBR and Fe3O4, as observed by colorimetry. It was characterized using high-resolution scanning electron microscopy (HRSEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared (FTIR) spectroscopy, powder X-ray diffraction (PXRD), and thermogravimetric analysis (TGA). Its catalytic activity was optimized by varying different parameters, and the optimum conditions for peroxidase mimetic activity were observed using 100 μL Fe3O4-GBR (1 mg/mL), 50 μL TMB (1 mg/mL), and 200 μL H2O2(1 mM) in 400 μL of acetate buffer of pH 2.3 at 30 °C temperature. Kinetic analysis has revealed the Michaelis–Menten kinetic behavior of peroxidase activity with Michaelis–Menten constants (Km) and maximum initial velocities (Vmax) of 0.082 mM and 14.1 nMs−1 respectively, for H2O2 and 0.086 mM and 5.1 nMs−1, respectively for TMB. The limit of detection and linear range were found to be 49.6 μM and 100–880 μM, respectively, for H2O2 and 41.9 μM and 47.6–952.3 μM, respectively, for cholesterol. On this basis, a simple, swift, sensitive, selective, and reproducible colorimetric assay to detect cholesterol levels in blood serum samples using Fe3O4-GBR nanocomposite has been developed. Thus, Fe3O4-GBR composite as compared to Fe3O4 and GBR has shown better peroxidase mimicking activity for biosensing.
Graphical abstract













Similar content being viewed by others
References
Wang J. Amperometric biosensors for clinical and therapeutic drug monitoring: a review. Pharmaceut Biomed. 1999. https://doi.org/10.1016/s0731-7085(98)00056-9.
Bakker E, Telting-Diaz M. Electrochemical sensors. Anal Chem. 2002. https://doi.org/10.1021/ac0202278.
Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008. https://doi.org/10.1021/cr068069y.
Libby P. The atherosclerosis new view. Sci Am. 2002. https://doi.org/10.1038/scientificamerican0502-46.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002. https://doi.org/10.1161/hc0902.104353.
Tang D, Niessner R, Knopp D. Flow-injection electrochemical immunosensor for the detection of human IgG based on glucose oxidase-derivated biomimetic interface. Biosens Bioelectron. 2009. https://doi.org/10.1016/j.bios.2008.11.008.
Koh WCA, Choe ES, Lee DK, Chang SC, Shim YB. Monitoring the activation of neuronal nitric oxide synthase in brain tissue and cells with a potentiometric immunosensor. Biosens Bioelectron. 2009. https://doi.org/10.1016/j.bios.2009.06.039.
Dzyadevych SV, Soldatkin AP, Arkhypova VN, Elskaya AV, Chovelon JM, Georgiou CA, Martelet C, Jaffrezic-Renault N. Early-warning electrochemical biosensor system for environmental monitoring based on enzyme inhibition. Sensor Actuat B-chem. 2005. https://doi.org/10.1016/j.snb.2004.02.039.
Walsh FC. Electrochemical technology for environmental treatment and clean energy conversion. Pure Appl Chem. 2001. https://doi.org/10.1351/pac200173121819.
Garg B, Bisht T, Ling Y-C. Graphene-based Nanomaterials as efficient peroxidase mimetic catalysts for biosensing applications: an overview. Molecules. 2015. https://doi.org/10.3390/molecules200814155.
Xie J, Cao H, Jiang H, Chen Y, Shi W, Zheng H, Huang Y. Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal ChimActa. 2013. https://doi.org/10.1016/j.aca.2013.08.008.
Kaushik A, Khan R, Solanki PR, Pandey P, Alam J, Ahmad S, Malhotra BD. Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens Bioelectron. 2008. https://doi.org/10.1016/j.bios.2008.06.032.
Kaushik A, Solanki PR, Ansari AA, Ahmad S, Malhotra BD. Chitosan–iron oxide nanobiocomposite based immunosensor for ochratoxin-a. ElectrochemCommun. 2008. https://doi.org/10.1016/j.elecom.2008.07.007.
Wang SF, Tan YM, Zhao DM, Liu GD. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles–chitosan nanocomposite. Biosens Bioelectron. 2008. https://doi.org/10.1016/j.bios.2008.02.014.
Hao J, Zhang Z, Yang W, Lu B, Ke X, Zhang B, Tang J. In situ controllable growth of CoFe2O4 ferrite nanocubes on graphene for colorimetric detection of hydrogen peroxide. J Mater Chem A. 2013. https://doi.org/10.1039/C3TA00774J.
Dong Y, Zhang H, Rahman ZU, Su L, Chen X, Hu J, Chen X. Graphene oxide–Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale. 2012. https://doi.org/10.1039/c2nr12109c.
Wang Q, Zhang X, Huang L, Zhang Z, Dong S. One-pot synthesis of Fe3O4 nanoparticle-loaded 3D porous graphene nanocomposites with enhanced nanozyme activity for glucose detection. ACS Appl Mater Interfaces. 2017. https://doi.org/10.1021/acsami.6b16034.
Zheng X, Liu Q, Jing C, Li Y, Li D, Luo W, Wen Y, He Y, Huang Q, Long YT, Fan C. Catalytic Gold Nanoparticles for Nanoplasmonic Detection of DNA Hybridization. Angew Chem Int Ed. 2011. https://doi.org/10.1002/anie.201105121.
He WW, Liu Y, Yuan JS, Yin JJ, Wu XC, Hu XN, Zhang K, Liu JB, Chen CY, Ji YL, Guo YT. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials. 2011. https://doi.org/10.1016/j.biomaterials.2010.09.040.
He W, Wu X, Liu J, Hu X, Zhang K, Hou S, Zhou W, Xie S. Design of AgM bimetallic alloy nanostructures (M = au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem Mater. 2010. https://doi.org/10.1021/cm100393v.
Zhao K, Gu W, Zheng SS, Zhang CL, Xian YZ. SDS–MoS2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H2O2 and glucose. Talanta. 2015. https://doi.org/10.1016/j.talanta.2015.03.055.
Chen W, Chen J, Feng YB, Hong L, Chen QY, Wu LF, Lin XH, Xia XH. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst. 2012. https://doi.org/10.1039/c2an35072f.
Nirala NR, Abraham S, Kumar V, Bansal A, Srivastava A, Saxena PS. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sensors Actuat B- Chem. 2015. https://doi.org/10.1016/j.snb.2015.04.091.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnology. 2007. https://doi.org/10.1038/nnano.2007.260.
Liu Y, Yuan M, Qiao L, Guo R. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens Bioelectron. 2014. https://doi.org/10.1016/j.bios.2013.09.020.
Liu Q, Li H, Zhao Q, Zhu R, Yang Y, Jia Q, Bian B, Zhuo L. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of porphyrin-Fe3O4 nanocomposites. Mater Sci Eng C Mater Biol Appl. 2014. https://doi.org/10.1016/j.msec.2014.04.038.
Li Q, Tang G, Xiong X, Cao Y, Chen L, Xu F, Tan H. Carbon coated magnetite nanoparticles with improved water-dispersion and peroxidase-like activity for colorimetric sensing of glucose sensors Actuat. B- Chem. 2015. https://doi.org/10.1016/j.snb.2015.03.029.
Yang W, Weng C, Li X, He H, Fei J, Xu W, Yan X, Zhu W, Zhang H, Zhou X. A sensitive colorimetric sensor based on one-pot preparation of h-Fe3O4@ppy with high peroxidase-like activity for determination of glutathione and H2O2. Sensors Actuat B- Chem. 2021. https://doi.org/10.1016/j.snb.2021.129844.
Abdelhamid HN, Mahmoud GAE, Sharmoukh W. A cerium-based MOFzyme with multi-enzyme-like activity for the disruption and inhibition of fungal recolonization. J Mater Chem B. 2020. https://doi.org/10.1039/d0tb90139c.
Abdelhamid HN, Sharmoukh W. Intrinsic catalase-mimicking MOFzyme for sensitive detection of hydrogen peroxide and ferric ions. Microchem J. 2021. https://doi.org/10.1016/j.microc.2020.105873.
Zenga J, Tangb Z, Li G. TMB colorimetric cholesterol biosensor based on reduced graphene oxide-Hemin nanocomposites IOP conf series. Earth Environ Sci. 2019. https://doi.org/10.1088/1755-1315/252/2/022134.
Qian J, Yang X, Yang Z, Zhu G, Mao H, Wang K. Multiwalled carbon nanotube@reducedgraphene oxide nanoribbon heterostructure: synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. J Mater Chem B. 2015. https://doi.org/10.1039/c4tb01702a.
Singh S, Mitra K, Shukla A, Singh R, Gundampati RK, Misra N, Maiti P, Ray B. Brominated Graphene as mimetic peroxidase for sulfide ion recognition. Anal Chem. 2017. https://doi.org/10.1021/acs.analchem.6b03535.
Singh S, Mitra K, Singh R, Kumari A, Gupta SKS, Mishra N, Maiti P, Ray B. Colorimetric detection of hydrogen peroxide and glucose using brominated Graphene. Anal Method. 2017. https://doi.org/10.1039/C7AY02212C.
Stoward PJ, Bradbury S. A note on the use of p-fluorophenylhydrazine for the cytochemical demonstration of periodate-reactive mucosaccharides and polysaccharides in the electron microscope. Histochemie. 1968. https://doi.org/10.1007/BF00304578.
Markovic OS, Pesic MP, Shah AV, Serajuddin ATM, Verbic TZ, Avdeef A. Solubility-pH profile of desipramine hydrochloride in saline phosphate buffer: enhanced solubility due to drug-buffer aggregates. Eur J Pharm Sci. 2019. https://doi.org/10.1016/j.ejps.2019.03.014.
See T, Pandikumar A, Ming H, Ngee L, Sulaiman Y. Simultaneous electrochemical detection of dopamine and ascorbic acid using an Iron oxide/reduced Graphene oxide modified glassy carbon electrode. Sensors. 2014. https://doi.org/10.3390/s140815227.
Teo PS, Lim HN, Huang NM, Chia CH, Harrion I. Room temperature in situ chemical synthesis of Fe3O4/graphene. Ceram Int. 2012. https://doi.org/10.1016/j.ceramint.2012.05.014.
Zhang M, Jia M, Jin Y. Fe3O4/reduced graphene oxide nanocomposite as high performance anode for lithium ion batteries. Appl Surf Sci. 2012. https://doi.org/10.1016/j.apsusc.2012.08.004.
Li Y, Zhang R, Tian X, Yang C, Zhou Z. Facile synthesis of Fe3O4 nanoparticles decorated on 3D graphene aerogels as broad-spectrum sorbents for water treatment. Appl Surf Sci. 2016. https://doi.org/10.1016/j.apsusc.2016.02.019.
Sharma V, Mobin SM. Cytocompatible peroxidase mimic CuO:graphene nanosphere composite as colorimetric dual sensor for hydrogen peroxide and cholesterol with its logic gate implementation. Sensors Actuat. B- Chem. 2017. https://doi.org/10.1016/j.snb.2016.08.169.
Chen J, Ge J, Zhange L, Li Z, Zhoua S, Qu L. PSS-GN nanocomposites as highly-efficient peroxidase mimic and their application to colorimetric detection of glucose in serum. RSC Adv. 2015. https://doi.org/10.1039/C5RA15837K.
Wu Y, Ma Y, Xu G, Wei F, Ma Y, Song Q, Wang X, Tang T, Song Y, Shi M, Xu X, Hu Q. Metal-organic framework coated Fe3O4 magnetic nanoparticles with peroxidase-like activity for colorimetric sensing of cholesterol. Sensors Actuat B- Chem. 2017. https://doi.org/10.1016/j.snb.2017.03.145.
Ren H, Ma T, Zhao J, Zhou R. Vc-functionalized Fe3O4Nanocomposites as peroxidase-like Mimetics for H2O2 and glucose sensing. Chem Res Chin Univ. 2018. https://doi.org/10.1007/s40242-018-7289-9.
Chen ZW, YinJ J, Zhou YT, Zhang Y, Song L, Song MJ, Hu SL, Gu N. Dual enzyme-like activities of Iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012. https://doi.org/10.1021/nn300291r.
Fan J, Yin JJ, Ning B, Wu XC, Hu Y, Ferrari M, Anderson GJ, Wei JY, Zhao YL, Nie GJ. Direct evidence for catalase and peroxidase activities of ferritin-platinum Nanoparticles. Biomaterials. 2011. https://doi.org/10.1016/j.biomaterials.2010.11.004.
Singh S, Singh M, Mitra K, Singh R, Gupta SKS, Tiwari I, Ray B. Electrochemical sensing of hydrogen peroxide using brominated graphene as mimetic catalase. Electrochim Acta. 2017. https://doi.org/10.1016/j.electacta.2017.12.006.
Acknowledgements
BR acknowledges the partial financial support from DBT (Govt. of India) through grant no. BT/PR889/NNT/28/570/2011 and the Council of Science and Industrial Research, Government of India, through Grant no. 02(0002)/11/EMR-II and IOE(BHU)(6031). RS, SV, and AK gratefully acknowledge the financial support from Banaras Hindu University through UGC-CRET-fellowship. Authors acknowledge Prof. R. K. Singh (Dept. of Physics, BHU) for providing the Raman spectroscopy facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Blood serum samples were collected from the Faculty of Ayurveda, Institute of Medical Science, Banaras Hindu University. All experiments related to blood serum were performed in compliance with the relevant laws and guidelines of the abovementioned institute.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1084 kb)
Rights and permissions
About this article
Cite this article
Singh, J., Singh, R., Singh, S. et al. Colorimetric detection of hydrogen peroxide and cholesterol using Fe3O4-brominated graphene nanocomposite. Anal Bioanal Chem 414, 2131–2145 (2022). https://doi.org/10.1007/s00216-021-03848-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03848-w