Abstract
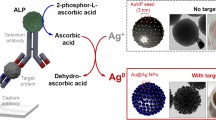
We developed a triple-readout probe for colorimetric, fluorescent, and fluorescence-lifetime sensing of alkaline phosphatase (ALP) through the hydrolyzed ascorbic acid phosphate (AAP)–mediated formation of silver nanoparticles (AgNPs) on Ag+-deposited MoS2 quantum dots (QDs). Ag+ ions were self-assembled on a monolayer MoS2 QD surface through the formation of Ag–S bonds. When ALP hydrolyzed AAP in an alkaline buffer, the resultant ascorbic acid (AA) triggered the reduction of the bound Ag+ ions into AgNPs on the MoS2 QD surface. The resultant AgNPs induced an efficient fluorescence quenching of the MoS2 QDs through simultaneous static and dynamic quenching processes, generated an intense surface plasmon resonance peak, and triggered a reduction in the fluorescence lifetime of the MoS2 QDs. Electron microscopy and spectroscopic techniques revealed the successful fabrication of Ag+-deposited MoS2 QDs and the ALP-mediated formation of AgNPs on the MoS2 QD surface. The linear quantification ranges for ALP were 0.05–2.5, 0.1–4, and 1–4 units L−1 in the fluorescent, colorimetric, and fluorescence-lifetime detection modes, respectively. In addition, the proposed probe integrated with an ALP-linked sandwich immunoassay exhibited high sensitivity and selectivity for the fluorescence sensing of rabbit immunoglobulin G with a detection limit of 8 pg mL−1 and linear range of 25–1000 pg mL−1. The sensitivity of the probe is comparable to those of previously reported immunoassays involving ultrasensitive electrochemical detection, hydrogen evolution reactions, or electron spin resonance. The probe integrated with the sandwich assay serves as a promising platform for the detection of target proteins in clinical samples.
Graphical abstract





Similar content being viewed by others
References
Zhang L, Buchet R, Azzar G. Phosphate binding in the active site of alkaline phosphatase and the interactions of 2-nitrosoacetophenone with alkaline phosphatase-induced small structural changes. Biophys J. 2004;86(6):3873–81.
Nikolic-Hughes I, O’brien PJ, Herschlag D. Alkaline phosphatase catalysis is ultrasensitive to charge sequestered between the active site zinc ions. J Am Chem Soc. 2005;127(26):9314–5.
Hausamen TU, Helger R, Rick W, Gross W. Optimal conditions for the determination of serum alkaline phosphatase by a new kinetic method. Clin Chim Acta. 1967;15(2):241–5.
Abdallah EAA, Said RN, Mosallam DS, Moawad EMI, Kamal NM, Fathallah MGE. Serial serum alkaline phosphatase as an early biomarker for osteopenia of prematurity. Medicine (Baltimore). 2016;95(37):e4837
Li D, Lv H, Hao X, Hu B, Song Y. Prognostic value of serum alkaline phosphatase in the survival of prostate cancer: evidence from a meta-analysis. Cancer Manag Res. 2018;10:3125.
Chen WZ, Shen JF, Zhou Y, Chen XY, Liu JM, Liu ZL. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci Rep. 2017;7(1):1–7.
Chen SC, Tsai SP, Jhao JY, Jiang WK, Tsao CK, Chang LY. Liver fat, hepatic enzymes, alkaline phosphatase and the risk of incident type 2 diabetes: a prospective study of 132,377 adults. Sci Rep. 2017;7(1):1–9.
Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Can Med Assoc J. 2005;172(3):367–79.
Lucarelli F, Marrazza G, Mascini M. Dendritic-like streptavidin/alkaline phosphatase nanoarchitectures for amplified electrochemical sensing of DNA sequences. Langmuir. 2006;22(9):4305–9.
Florentinus-Mefailoski A, Marshall JG. Linear quantification of a streptavidin–alkaline phosphatase probe for enzyme-linked immuno mass spectrometric assay. Anal Biochem. 2016;503:50–5.
Selvolini G, Lettieri M, Tassoni L, Gastaldello S, Grillo M, Maran C, et al. Electrochemical enzyme-linked oligonucleotide array for aflatoxin B1 detection. Talanta. 2019;203:49–57.
Sun Z, Wang X, Chen Q, Yun Y, Tang Z, Liu X. Nanobody-alkaline phosphatase fusion protein-based enzyme-linked immunosorbent assay for one-step detection of ochratoxin A in rice. Sensors. 2018;18(11):4044.
Gao Z, Hou L, Xu M, Tang D. Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci Rep. 2014;4(1):1–8.
Liu Y, Schanze KS. Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal Chem. 2008;80(22):8605–12.
Zhang L, Zhao J, Duan M, Zhang H, Jiang J, Yu R. Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal Chem. 2013;85(8):3797–801.
Bhimji A, Zaragoza AA, Live LS, Kelley SO. Electrochemical enzyme-linked immunosorbent assay featuring proximal reagent generation: detection of human immunodeficiency virus antibodies in clinical samples. Anal Chem. 2013;85(14):6813–9.
Liu H, Wei L, Hua J, Chen D, Meng H, Li Z, et al. Enzyme activity-modulated etching of gold nanobipyramids@ MnO 2 nanoparticles for ALP assay using surface-enhanced Raman spectroscopy. Nanoscale. 2020;12(18):10390–8.
Han Y, Chen J, Li Z, Chen H, Qiu H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens Bioelectron. 2020;148:111811.
Kong RM, Fu T, Sun NN, Qu FL, Zhang SF, Zhang XB. Pyrophosphate-regulated Zn2+-dependent DNAzyme activity: An amplified fluorescence sensing strategy for alkaline phosphatase. Biosens Bioelectron. 2013;50:351–5.
Chen C, Yuan Q, Ni P, Jiang Y, Zhao Z, Lu Y. Fluorescence assay for alkaline phosphatase based on ATP hydrolysis-triggered dissociation of cerium coordination polymer nanoparticles. Analyst. 2018;143(16):3821–8.
Cao XY, Kong FZ, Zhang Q, Liu WW, Liu XP, Li GQ, et al. iPhone-imaged and cell-powered electrophoresis titration chip for the alkaline phosphatase assay in serum by the moving reaction boundary. Lab Chip. 2018;18(12):1758–66.
Liu H, Li M, Xia Y, Ren X. A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mater Interfaces. 2017;9(1):120–6.
Chen P, Yan S, Sawyer E, Ying B, Wei X, Wu Z, et al. Rapid and simple detection of ascorbic acid and alkaline phosphatase via controlled generation of silver nanoparticles and selective recognition. Analyst. 2019;144(4):1147–52.
Wang F, Li Y, Li W, Zhang Q, Chen J, Zhou H, et al. A facile method for detection of alkaline phosphatase activity based on the turn-on fluorescence of resorufin. Anal Methods. 2014;6(15):6105–9.
Zhao D, Li J, Peng C, Zhu S, Sun J, Yang X. Fluorescence immunoassay based on the alkaline phosphatase triggered in situ fluorogenic reaction of o-phenylenediamine and ascorbic acid. Anal Chem. 2019;91(4):2978–84.
Sun J, Hu T, Chen C, Zhao D, Yang F, Yang X. Fluorescence immunoassay system via enzyme-enabled in situ synthesis of fluorescent silicon nanoparticles. Anal Chem. 2016;88(19):9789–95.
Hu Q, Zhou B, Li F, Kong J, Zhang X. Turn-on colorimetric platform for dual activity detection of acid and alkaline phosphatase in human whole blood. Chem Asian J. 2016;11(21):3040–5.
Hu L, Zhang Q, Gan X, Lin S, Han S, Zhang Z. Fluorometric turn-on determination of the activity of alkaline phosphatase by using WS 2 quantum dots and enzymatic cleavage of ascorbic acid 2-phosphate. Microchim Acta. 2018;185(8):1–6.
Ni P, Chen C, Jiang Y, Zhang C, Wang B, Cao B, et al. Gold nanoclusters-based dual-channel assay for colorimetric and turn-on fluorescent sensing of alkaline phosphatase. Sens Actuators B Chem. 2019;301:127080.
Liang MY, Zhao B, Xiong Y, Chen WX, Huo JZ, Zhang F, et al. A “turn-on” sensor based on MnO 2 coated UCNPs for detection of alkaline phosphatase and ascorbic acid. Dalton Trans. 2019;48(43):16199–210.
Pham XH, Hahm E, Kim TH, Kim HM, Lee SH, Lee YS, et al. Enzyme-catalyzed Ag growth on Au nanoparticle-assembled structure for highly sensitive colorimetric immunoassay. Sci Rep. 2018;8(1):1–7.
He Y, Jiao B. Determination of the activity of alkaline phosphatase based on the use of ssDNA-templated fluorescent silver nanoclusters and on enzyme-triggered silver reduction. Microchim Acta. 2017;184(10):4167–73.
Wang H, Yang L, Chu S, Liu B, Zhang Q, Zou L, et al. Semiquantitative visual detection of lead ions with a smartphone via a colorimetric paper-based analytical device. Anal Chem. 2019;91(14):9292–9.
Wang H, Da L, Yang L, Chu S, Yang F, Yu S, et al. Colorimetric fluorescent paper strip with smartphone platform for quantitative detection of cadmium ions in real samples. J Hazard Mater. 2020;392:122506.
Chu S, Wang H, Ling X, Yu S, Yang L, Jiang C. A portable smartphone platform using a ratiometric fluorescent paper strip for visual quantitative sensing. ACS Appl Mater Interfaces. 2020;12(11):12962–71.
Chu S, Wang H, Du Y, Yang F, Yang L, Jiang C. Portable smartphone platform integrated with a nanoprobe-based fluorescent paper strip: visual monitoring of glutathione in human serum for health prognosis. ACS Sustain Chem Eng. 2020;8(22):8175–83.
Liu SG, Han L, Li N, Xiao N, Ju YJ, Li NB, et al. A fluorescence and colorimetric dual-mode assay of alkaline phosphatase activity via destroying oxidase-like CoOOH nanoflakes. J Mater Chem B. 2018;6(18):2843–50.
Zhao J, Wang S, Lu S, Bao X, Sun J, Yang X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal Chem. 2018;90(12):7754–60.
Zhao J, Wang S, Lu S, Liu G, Sun J, Yang X. Fluorometric and colorimetric dual-readout immunoassay based on an alkaline phosphatase-triggered reaction. Anal Chem. 2019;91(12):7828–34.
Chen C, Zhang G, Ni P, Jiang Y, Lu Y, Lu Z. Fluorometric and colorimetric dual-readout alkaline phosphatase activity assay based on enzymatically induced formation of colored Au@ Ag nanoparticles and an inner filter effect. Microchim Acta. 2019;186(6):348.
Zhang J, He L, Zhang X, Wang J, Yang L, Liu B, et al. Colorimetric and SERS dual-readout for assaying alkaline phosphatase activity by ascorbic acid induced aggregation of Ag coated Au nanoparticles. Sens Actuators B Chem. 2017;253:839–45.
Gopalakrishnan D, Damien D, Shaijumon MM. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano. 2014;8(5):5297–303.
Xu G, Yang L, Wei X, Ding J, Zhong J, Chu PK. MoS2-Quantum-Dot-Interspersed Li4Ti5O12 Nanosheets with Enhanced Performance for Li-and Na-Ion Batteries. Adv Funct Mater. 2016;26(19):3349–58.
Gu W, Yan Y, Zhang C, Ding C, Xian Y. One-step synthesis of water-soluble MoS2 quantum dots via a hydrothermal method as a fluorescent probe for hyaluronidase detection. ACS Appl Mater Interfaces. 2016;8(18):11272–9.
Yue N, Weicheng J, Rongguo W, Guomin D, Yifan H. Hybrid nanostructures combining graphene–MoS 2 quantum dots for gas sensing. J Mater Chem A. 2016;4(21):8198–203.
Chen SC, Lin CY, Cheng TL, Tseng WL. 6-mercaptopurine-induced fluorescence quenching of monolayer MoS2 nanodots: applications to glutathione sensing, cellular imaging, and glutathione-stimulated drug delivery. Adv Funct Mater. 2017;27(41):1702452.
Wang Z, Sim A, Urban JJ, Mi B. Removal and recovery of heavy metal ions by two-dimensional MoS2 nanosheets: performance and mechanisms. Environ Sci Technol. 2018;52(17):9741–8.
Wu MJ, Tseng WL. Rapid, facile, reagentless, and room-temperature conjugation of monolayer MoS 2 nanosheets with dual-fluorophore-labeled flares as nanoprobes for ratiometric sensing of TK1 mRNA in living cells. J Mater Chem B. 2020;8(8):1692–8.
Kumar ASK, Tseng W-B, Wu MJ, Huang YY, Tseng WL. L-cystine-linked BODIPY-adsorbed monolayer MoS2 quantum dots for ratiometric fluorescent sensing of biothiols based on the inner filter effect. Anal Chim Acta. 2020;1113:43–51.
Lin JH, Yang YC, Shih YC, Hung SY, Lu CY, Tseng WL. Photoinduced electron transfer between Fe (III) and adenosine triphosphate-BODIPY conjugates: application to alkaline-phosphatase-linked immunoassay. Biosens Bioelectron. 2016;77:242–8.
Li B, Jiang L, Li X, Ran P, Zuo P, Wang A, et al. Preparation of monolayer MoS 2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS 2 targets in water. Sci Rep. 2017;7(1):1–12.
Cao F, Ju E, Zhang Y, Wang Z, Liu C, Li W, et al. An efficient and benign antimicrobial depot based on silver-infused MoS2. ACS Nano. 2017;11(5):4651–9.
Lei Y, Li D, Zhang TC, Huang X, Liu L, Lu Y. One-step selective formation of silver nanoparticles on atomic layered MoS 2 by laser-induced defect engineering and photoreduction. J Mater Chem C. 2017;5(34):8883–92.
Zhou J, Chen J, Ge Y, Shao Y. Two-dimensional nanomaterials for Förster resonance energy transfer–based sensing applications. Nanophotonics. 2020;9(7):1855–75.
Guo X, Huang J, Zeng Q, Wei Y, Liu X, Wang L. Boronic acid-functionalized molybdenum disulfide quantum dots for the ultrasensitive analysis of dopamine based on synergistic quenching effects from IFE and aggregation. J Mater Chem B. 2019;7(17):2799–807.
Paramelle D, Sadovoy A, Gorelik S, Free P, Hobley J, Fernig DG. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst. 2014;139(19):4855–61.
Zhong Y, Yi T. MoS 2 quantum dots as a unique fluorescent “turn-off–on” probe for the simple and rapid determination of adenosine triphosphate. J Mater Chem B. 2019;7(15):2549–56.
Raghavendra U, Thipperudrappa J, Basanagouda M, Melavanki R. Influence of silver nanoparticles on spectroscopic properties of biologically active iodinated 4-aryloxymethyl coumarin dyes. J Lumin. 2016;172:139–46.
Gambucci M, Tarpani L, Zampini G, Massaro G, Nocchetti M, Sassi P, et al. Fluorimetric studies of a transmembrane protein and its interactions with differently functionalized silver nanoparticles. J Phys Chem B. 2018;122(27):6872–9.
Deng H, Yu H. Silver nanoparticle surface enabled self-assembly of organic dye molecules. Materials. 2019;12(16):2592.
Han Y, Niu Y, Liu M, Niu F, Xu Y. A rational strategy to develop a boron nitride quantum dot-based molecular logic gate and fluorescent assay of alkaline phosphatase activity. J Mater Chem B. 2019;7(6):897–902.
Niu F, Ying YL, Hua X, Niu Y, Xu Y, Long YT. Electrochemically generated green-fluorescent N-doped carbon quantum dots for facile monitoring alkaline phosphatase activity based on the Fe3+-mediating ON-OFF-ON-OFF fluorescence principle. Carbon. 2018;127:340–8.
Zhong Y, Xue F, Wei P, Li R, Cao C, Yi T. Water-soluble MoS 2 quantum dots for facile and sensitive fluorescence sensing of alkaline phosphatase activity in serum and live cells based on the inner filter effect. Nanoscale. 2018;10(45):21298–306.
Na W, Li N, Xingguang S. Enzymatic growth of single-layer MnO2 nanosheets in situ: application to detect alkaline phosphatase and ascorbic acid in the presence of sulfanilic acid functionalized graphene quantum dots. Sens Actuators B Chem. 2018;274:172–9.
Zhang F, He X, Ma P, Sun Y, Wang X, Song D. Rapid aqueous synthesis of CuInS/ZnS quantum dots as sensor probe for alkaline phosphatase detection and targeted imaging in cancer cells. Talanta. 2018;189:411–7.
Shi D, Sun Y, Lin L, Shi C, Wang G, Zhang X. Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu (ii). Analyst. 2016;141(19):5549–54.
Xianyu Y, Wang Z, Jiang X. A plasmonic nanosensor for immunoassay via enzyme-triggered click chemistry. ACS Nano. 2014;8(12):12741–7.
Gao Z, Deng K, Wang X-D, Miró M, Tang D. High-resolution colorimetric assay for rapid visual readout of phosphatase activity based on gold/silver core/shell nanorod. ACS Appl Mater Interfaces. 2014;6(20):18243–50.
Xiao T, Sun J, Zhao J, Wang S, Liu G, Yang X. FRET effect between fluorescent polydopamine nanoparticles and MnO2 nanosheets and its application for sensitive sensing of alkaline phosphatase. ACS Appl Mater Interfaces. 2018;10(7):6560–9.
Acknowledgements
We would like to thank the Ministry of Science and Technology of Taiwan (MOST107-2113-M-110-013-MY3; MOST 110-2113-M-017-002-MY3) and NSYSU-KMU Joint Research Project (NSYSUKMU 109-P002) for the financial support of this study.
Author information
Authors and Affiliations
Contributions
Manivannan Madhu: Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. Chien-Min Chao: Investigation, Formal analysis. Chen-Yi Ke: Investigation, Formal analysis, Visualization. Ming-Mu Hsieh: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition. Wei-Lung Tseng: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Conflicts of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madhu, M., Chao, CM., Ke, CY. et al. Directed self-assembly of Ag+-deposited MoS2 quantum dots for colorimetric, fluorescent and fluorescence-lifetime sensing of alkaline phosphatase. Anal Bioanal Chem 414, 1909–1919 (2022). https://doi.org/10.1007/s00216-021-03826-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03826-2