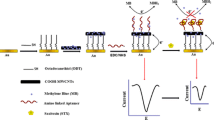
Abstract
Saxitoxin is a cyanotoxin which is very harmful to human health; the concentration limit in drinking water is only 3 μg/L. Therefore, a simple, fast, sensitive, low-cost, and specific method for its detection, quantification, and monitoring in water bodies is needed to avoid adverse effects on animal and human health. In this work, we developed an electrochemical impedimetric biosensor using a specific aptamer as recognition element for saxitoxin detection. This method allies the superior sensing characteristics of aptamers with the nondestructive, label-free, and easy working principles of the electrochemical impedance technique. The device presented sensitivity for detecting saxitoxin concentrations above 0.3 μg/L, with high selectivity in negative control experiments, demonstrating a promising alternative for water toxin detection.
Graphical abstract





Similar content being viewed by others
References
Chorus I, Welker M, editors. Toxic cyanobacteria in water. 2nd ed. Oxfordshire: Taylor & Francis; 2021.
Zhang W, Dixon MB, Saint C, Teng KS, Furumai H. Electrochemical biosensing of algal toxins in water: the current state-of-the-art. ACS Sens. 2018;3(7):1233–45. https://doi.org/10.1021/acssensors.8b00359.
Munoz M, Nieto-Sandoval J, Cirés S, de Pedro ZM, Quesada A, Casas JA. Degradation of widespread cyanotoxins with high impact in drinking water (microcystins, cylindrospermopsin, anatoxin-a and saxitoxin) by CWPO. Water Res. 2019;163(10):114853. https://doi.org/10.1016/j.watres.2019.114853.
Azevedo SMFO, et al. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology. 2002;181–182(12):441–6. https://doi.org/10.1016/S0300-483X(02)00491-2.
Baptista M, Nixdorf B. Low disturbances favor steady state: case of cyanobacterial monodominance in a Brazilian coastal lagoon. Inl Waters. 2014;4(2):243–54. https://doi.org/10.5268/IW-4.2.648.
Ramos TK, et al. Saxitoxins from the freshwater cyanobacterium Raphidiopsis raciborskii can contaminate marine mussels. Harmful Algae. 2021;103(3):102004. https://doi.org/10.1016/j.hal.2021.102004.
Bratakou S, Nikoleli G-P, Siontorou CG, Nikolelis DP, Karapetis S, Tzamtzis N. Development of an electrochemical biosensor for the rapid detection of saxitoxin based on air stable lipid films with incorporated anti-STX using graphene electrodes. Electroanalysis. 2017;29(4):990–7. https://doi.org/10.1002/elan.201600652.
Hou L, et al. Amperometric aptasensor for saxitoxin using a gold electrode modified with carbon nanotubes on a self-assembled monolayer, and methylene blue as an electrochemical indicator probe. Microchim Acta. 2016;183(6):1971–80. https://doi.org/10.1007/s00604-016-1836-1.
Gao S, Zheng X, Wu J. A biolayer interferometry-based competitive biosensor for rapid and sensitive detection of saxitoxin. Sensors Actuators B Chem. 2017;246(7):169–74. https://doi.org/10.1016/j.snb.2017.02.078.
Cheng S, et al. Study of the binding way between saxitoxin and its aptamer and a fluorescent aptasensor for detection of saxitoxin. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;204:180–7. https://doi.org/10.1016/J.SAA.2018.06.036.
Ye W, et al. Marine toxins detection by biosensors based on aptamers. Toxins. 2019;12(1):1. https://doi.org/10.3390/toxins12010001.
Cheng J, et al. A new simple screening method for the detection of paralytic shellfish poisoning toxins. Chin J Oceanol Limnol. 2012;30(5):786–90. https://doi.org/10.1007/s00343-012-1097-8.
Ling S, et al. Preparation of monoclonal antibody for brevetoxin 1 and development of Ic-ELISA and colloidal gold strip to detect brevetoxin 1. Toxins (Basel). 10(2):75. https://doi.org/10.3390/toxins10020075.
Halme M, Rapinoja ML, Karjalainen M, Vanninen P. Verification and quantification of saxitoxin from algal samples using fast and validated hydrophilic interaction liquid chromatography-tandem mass spectrometry method. J Chromatogr B Anal Technol Biomed Life Sci. 2012;880(1):50–7. https://doi.org/10.1016/j.jchromb.2011.11.015.
Krock B, et al. LC-MS/MS detection of karlotoxins reveals new variants in strains of the marine dinoflagellate karlodinium veneficum from the ebro delta (NW mediterranean). Mar Drugs. 2017;15(12):391. https://doi.org/10.3390/md15120391.
Zheng X, et al. A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon. 2015;101:41–7. https://doi.org/10.1016/j.toxicon.2015.04.017.
Handy SM, et al. First report of the use of a saxitoxin-protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon. 2013;61(1):30–7. https://doi.org/10.1016/j.toxicon.2012.10.015.
Lai W, Wei Q, Zhuang J, Lu M, Tang D. Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens Bioelectron. 2016;80:249–56. https://doi.org/10.1016/j.bios.2016.01.088.
Lai W, Zhuang J, Tang D. Novel colorimetric immunoassay for ultrasensitive monitoring of brevetoxin b based on enzyme-controlled chemical conversion of sulfite to sulfate. J Agric Food Chem. 2015;63(7):1982–9. https://doi.org/10.1021/acs.jafc.5b00425.
Lin Y, Zhou Q, Tang D. Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-doped Zn3(OH)2V2O7 nanobelts. Anal Chem. 2017;89(21):11803–10. https://doi.org/10.1021/acs.analchem.7b03451.
Lin Y, Zhou Q, Tang D, Niessner R, Knopp D. Signal-on photoelectrochemical immunoassay for aflatoxin B1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal Chem. 2017;89(10):5637–45. https://doi.org/10.1021/acs.analchem.7b00942.
Lin Y, Zhou Q, Zeng Y, Tang D. Liposome-coated mesoporous silica nanoparticles loaded with L-cysteine for photoelectrochemical immunoassay of aflatoxin B1. Microchim Acta. 2018;185(6):1–9. https://doi.org/10.1007/s00604-018-2848-9.
Bahadır EB, Sezgintürk MK. A review on impedimetric biosensors. Artif Cells Nanomed Biotechnol. 2016;44(1):248–62. https://doi.org/10.3109/21691401.2014.942456.
Xu M, Gao Z, Wei Q, Chen G, Tang D. Hemin/G-quadruplex-based DNAzyme concatamers for in situ amplified impedimetric sensing of copper(II) ion coupling with DNAzyme-catalyzed precipitation strategy. Biosens Bioelectron. 2015;74:1–7. https://doi.org/10.1016/j.bios.2015.05.056.
Qiu Z, Tang D, Shu J, Chen G, Tang D. Enzyme-triggered formation of enzyme-tyramine concatamers on nanogold-functionalized dendrimer for impedimetric detection of Hg(II) with sensitivity enhancement. Biosens Bioelectron. 2016;75:108–15. https://doi.org/10.1016/j.bios.2015.08.026.
Hou L, Wu X, Chen G, Yang H, Lu M, Tang D. HCR-stimulated formation of DNAzyme concatamers on gold nanoparticle for ultrasensitive impedimetric immunoassay. Biosens Bioelectron. 2015;68:487–93. https://doi.org/10.1016/j.bios.2015.01.043.
Kong HY, Byun J. Nucleic acid aptamers: new methods for selection, stabilization, and application in biomedical science. Biomolecul Therapeut. 2013;21(6):423–34. https://doi.org/10.4062/biomolther.2013.085.
Bazin I, Tria SA, Hayat A, Marty J-L. New biorecognition molecules in biosensors for the detection of toxins. Biosens Bioelectron. 2017;87:285–98. https://doi.org/10.1016/j.bios.2016.06.083.
Zhou W, Jimmy Huang P-J, Ding J, Liu J. Aptamer-based biosensors for biomedical diagnostics. Analyst. 2014;139(11):2627. https://doi.org/10.1039/c4an00132j.
Cruz-Aguado JA, Penner G. Determination of ochratoxin A with a DNA Aptamer. J Agric Food Chem. 2008;56(22):10456–61. https://doi.org/10.1021/jf801957h.
Eissa S, Ng A, Siaj M, Tavares AC, Zourob M. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal Chem. 2013;85(24):11794–801. https://doi.org/10.1021/ac402220k.
Ng A, Chinnappan R, Eissa S, Liu H, Tlili C, Zourob M. Selection, characterization, and biosensing application of high affinity congener-specific microcystin-targeting aptamers. Environ Sci Technol. 2012;46(19):10697–703. https://doi.org/10.1021/es301686k.
Alfaro K, Bustos P, O’Sullivan C, Conejeros P. Facile and cost-effective detection of saxitoxin exploiting aptamer structural switching. Food Technol Biotechnol. 2015;53(3):337–41. https://doi.org/10.17113/ftb.53.03.15.3911.
Zhong L, et al. Portable smartphone-based colorimetric analyzer with enhanced gold nanoparticles for on-site tests of seafood safety. Anal Sci. 2019;35(2):133–40. https://doi.org/10.2116/analsci.18P184.
Cheng S, et al. Determination of saxitoxin by aptamer-based surface-enhanced raman scattering. Anal Lett. 2019;52(6):902–18 https://doi.org/10.1080/00032719.2018.1505900.
Caglayan MO, Üstündağ Z. Saxitoxin aptasensor based on attenuated internal reflection ellipsometry for seafood. Toxicon. 2020;187:255–61. https://doi.org/10.1016/j.toxicon.2020.09.005.
Erdem A, Congur G. Dendrimer modified 8-channel screen-printed electrochemical array system for impedimetric detection of activated protein C. Sensors Actuators B Chem. 2014;196:168–74. https://doi.org/10.1016/j.snb.2014.01.103.
Elshafey R, Tavares AC, Siaj M, Zourob M. Electrochemical impedance immunosensor based on gold nanoparticles–protein G for the detection of cancer marker epidermal growth factor receptor in human plasma and brain tissue. Biosens Bioelectron. 2013;50:143–9. https://doi.org/10.1016/j.bios.2013.05.063.
Lin Z, et al. Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta. 2013;103:371–4. https://doi.org/10.1016/j.talanta.2012.10.081.
Elshafey R, Siaj M, Zourob M. DNA aptamers selection and characterization for development of label-free impedimetric aptasensor for neurotoxin anatoxin-a. Biosens Bioelectron. 2015;68:295–302. https://doi.org/10.1016/j.bios.2015.01.002.
Elshafey R, Siaj M, Zourob M. In vitro selection, characterization, and biosensing application of high-affinity cylindrospermopsin-targeting aptamers. Anal Chem. 2014;86(18):9196–203. https://doi.org/10.1021/ac502157g.
Lasia A. Electrochemical impedance spectroscopy and its applications. 1st ed. New York: Springer; 2014.
Yuan X-Z, Song C, Wang H, Zhang J. Electrochemical impedance spectroscopy in PEM fuel cells. London: Springer London; 2010.
Funding
This work received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), and Instituto Nacional de Ciência e Tecnologia em Eletrônica Orgânica (INCT/INEO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serrano, P.C., Nunes, G.E., Avila, L.B. et al. Electrochemical impedance biosensor for detection of saxitoxin in aqueous solution. Anal Bioanal Chem 413, 6393–6399 (2021). https://doi.org/10.1007/s00216-021-03603-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03603-1