Abstract
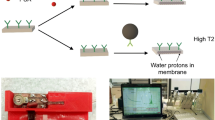
A nuclear magnetic resonance (NMR) immunoassay based on the application of carbon-coated iron nanoparticles conjugated with recognition molecules was designed. The principle of the assay is that ELISA plates are coated with a capture element, and then an analyte is added and detected by conjugating the magnetic nanoparticles with recognition molecules. Afterwards, the elution solution (0.1-M sodium hydroxide) is added to displace the magnetic nanoparticles from the well surfaces into the solution. The detached magnetic nanoparticles reduce transverse relaxation time (T2) values of protons from the surrounding solution. A portable NMR relaxometer is used to measure the T2. Magnetic nanoparticles conjugated with streptavidin, monoclonal antibodies, and protein G were applied for the detection of biotinylated albumin, prostate-specific antigen, and IgG specific to tetanus toxoid (TT). The limit of detection of anti-TT IgG was 0.08–0.12 mIU/mL. The reproducibility of the assay was within the acceptable range (CV < 7.4%). The key novelty of the immunoassay is that the displacement of the nanoparticles from the solid support by the elution solution allows the advantages of the solid phase assay to be combined with the sensitive detection of the T2 changes in a volume of liquid.
Graphical abstract






Similar content being viewed by others
References
Alcantara D, Lopez S, García-Martin ML, Pozo D. Iron oxide nanoparticles as magnetic relaxation switching (MRSw) sensors: current applications in nanomedicine. Nanomedicine. 2016;12:1253–62. https://doi.org/10.1016/j.nano.2016.01.005.
Kim J, Mohamed MAA, Zagorovsky K, Chan WCW. State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials. 2017;146:97–114. https://doi.org/10.1016/j.biomaterials.2017.08.013.
Denmark DJ, Bustos-Perez X, Swain A, Phan MH, Mohapatra S, Mohapatra SS. Readiness of magnetic nanobiosensors for point-of-care commercialization. J Electron Mater. 2019;48:4749–61. https://doi.org/10.1007/s11664-019-07275-7.
Huang Z, Hu S, Xiong Y, Wei H, Xu H, Duan H, et al. Application and development of superparamagnetic nanoparticles in sample pretreatment and immunochromatographic assay. Trends Anal Chem. 2019;114:151–70. https://doi.org/10.1016/j.trac.2019.03.004.
Knežević NŽ, Gadjanski I, Durand JO. Magnetic nanoarchitectures for cancer sensing, imaging and therapy. J Mater Chem B. 2019;7:9–23. https://doi.org/10.1039/c8tb02741b.
Nabaei V, Chandrawati R, Heidari H. Magnetic biosensors: modelling and simulation. Biosens Bioelectron. 2018;103:69–86. https://doi.org/10.1016/j.bios.2017.12.023.
Yang J, Wang K, Xu H, Yan W, Jin Q, Cui D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: a review. Talanta. 2019;202:96–110. https://doi.org/10.1016/j.talanta.2019.04.054.
Zhang Y, Yang H, Zhou Z, Huang K, Yang S, Han G. Recent advances on magnetic relaxation switching assay-based nanosensors. Bioconjug Chem. 2017;28:869–79. https://doi.org/10.1021/acs.bioconjchem.7b00059.
Ullman E. Homogeneous immunoassays. In: Wild D, editor. The immunoassay handbook. Amsterdam: Elsevier Science; 2013. p. 67–87.
Burtea C, Laurent S, Mahieu I, Larbanoix L, Roch A, Port M, et al. In vitro biomedical applications of functionalized iron oxide nanoparticles, including those not related to magnetic properties. Contrast Media Mol Imaging. 2011;6:236–50. https://doi.org/10.1002/cmmi.423.
Khramtsov P, Kropaneva M, Bochkova M, Timganova V, Zamorina S, Rayev M. Solid-phase nuclear magnetic resonance immunoassay for the prostate-specific antigen by using protein-coated magnetic nanoparticles. Microchim Acta. 2019;186:768. https://doi.org/10.1007/s00604-019-3925-4.
Khramtsov P, Barkina I, Kropaneva M, Bochkova M, Timganova V, Nechaev A, et al. Magnetic nanoclusters coated with albumin, casein, and gelatin: size tuning, relaxivity, stability, protein corona, and application in nuclear magnetic resonance immunoassay. Nanomaterials. 2019;9:1345. https://doi.org/10.3390/nano9091345.
Piletsky SS, Cass AEG, Piletska EV, Czulak J, Piletsky SA. A novel assay format as an alternative to ELISA: MINA test for biotin. ChemNanoMat. 2018;4:1214–22. https://doi.org/10.1002/cnma.201800393.
Mao X, Jiang J, Chen J, Huang Y, Shen G, Yu R. Cyclic accumulation of nanoparticles: a new strategy for electrochemical immunoassay based on the reversible reaction between dethiobiotin and avidin. Anal Chim Acta. 2006;557:159–63. https://doi.org/10.1016/j.aca.2005.09.078.
Glass JR, Dickerson JC, Schultz DA. Enzyme-mediated individual nanoparticle release assay. Anal Biochem. 2006;353:209–16. https://doi.org/10.1016/j.ab.2006.03.020.
Lin Y, Zhou Q, Lin Y, Tang D, Chen G, Tang D. Simple and sensitive detection of aflatoxin B1 within five minute using a non-conventional competitive immunosensing mode. Biosens Bioelectron. 2015;74:680–6. https://doi.org/10.1016/j.bios.2015.07.029.
Takae S, Akiyama Y, Yamasaki Y, Nagasaki Y, Kataoka K. Colloidal Au replacement assay for highly sensitive quantification of low molecular weight analytes by surface plasmon resonance. Bioconjug Chem. 2007;18:1241–5. https://doi.org/10.1021/bc0603541.
Halonen S, Kangas T, Haataja M, Lassi U. Urea-water-solution properties: density, viscosity, and surface tension in an under-saturated solution. Emiss Control Sci Technol. 2017;3:161–70. https://doi.org/10.1007/s40825-016-0051-1Y.K.
Agrawal YK, Sabbagh R, Sanders S, Nobes DS. Measuring the refractive index, density, viscosity, pH, and surface tension of potassium thiocyanate (KSCN) solutions for refractive index matching in flow experiments. J Chem Eng Data. 2018;63:1275–85. https://doi.org/10.1021/acs.jced.7b00904.
Warren JR, Gordon JA. On the refractive indices of aqueous solutions of urea. J Phys Chem. 1966;70:297–300. https://doi.org/10.1021/j100873a507.
Ayyar BV, Arora S, Murphy C, O'Kennedy R. Affinity chromatography as a tool for antibody purification. Methods. 2012;56:116–29. https://doi.org/10.1016/j.ymeth.2011.10.007.
Yan Z, Huang J. Chromatographic behavior of mouse serum immunoglobulin G in protein G perfusion affinity chromatography. Chromatogr B Biomed Sci Appl. 2000;738:149–54. https://doi.org/10.1016/S0378-4347(99)00507-1.
Acevedo B, Perera Y, Torres E, Pentón D, Ayala M, Gavilondo J. Fast and novel purification method to obtain the prostate specific antigen (PSA) from human seminal plasma. Prostate. 2006;66:1029–36. https://doi.org/10.1002/pros.20267.
Fahmi MZ, Ou K, Chen JK, Ho MH, Tzing SH, Chang JY. Development of bovine serum albumin-modified hybrid nanoclusters for magnetofluorescence imaging and drug delivery. RSC Adv. 2014;4:32762–72. https://doi.org/10.1039/c4ra05785f.
Strozyk MS, Chanana M, Pastoriza-Santos I, Pérez-Juste J, Liz-Marzán LM. Protein/polymer-based dual-responsive gold nanoparticles with pH-dependent thermal sensitivity. Adv Funct Mater. 2012;22:1436–44. https://doi.org/10.1002/adfm.201102471.
Rybak JN, Scheurer SB, Neri D, Elia G. Purification of biotinylated proteins on streptavidin resin: a protocol for quantitative elution. Proteomics. 2004;4:2296–9. https://doi.org/10.1002/pmic.200300780.
Yen LM, Thwaites CL. Tetanus. Lancet. 2019;393:1657–68. https://doi.org/10.1016/S0140-6736(18)33131-3.
Yilmaz A, Ulak FŞ, Batun MS. Proton T1 and T2 relaxivities of serum proteins. Magn Reson Imaging. 2004;22:683–8. https://doi.org/10.1016/j.mri.2004.02.001.
Kristiansen M, Aggerbeck H, Heron I. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS. 1997;105:843–53.
van Gageldonk PGM, van Schaijk FG, van der Klis FR, Berbers GAM. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008;335:79–89. https://doi.org/10.1016/j.jim.2008.02.018.
Liu J, Wang J, Li Z, Meng H, Zhang L, Wang H, et al. A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Microchim Acta. 2018;185:110. https://doi.org/10.1007/s00604-017-2657-6.
Raeisi S, Molaeirad A, Sadri M, Nejad HR. Detection of anti-tetanus toxoid monoclonal antibody by using modified polycarbonate surface. Plasmonics. 2018;13:1555–67. https://doi.org/10.1007/s11468-017-0664-4.
Jain S, Chattopadhyay S, Jackeray R, Zainul Abid CKV, Kumar M, Singh H. Detection of anti-tetanus toxoid antibody on modified polyacrylonitrile fibers. Talanta. 2010;82:1876–83. https://doi.org/10.1016/j.talanta.2010.08.003.
Golberg A, Yarmush ML, Konry T. Picoliter droplet microfluidic immunosorbent platform for point-of-care diagnostics of tetanus. Microchim Acta. 2013;180:855–60. https://doi.org/10.1007/s00604-013-0998-3.
Bioanalytical Method Validation. FDA. 2018. https://www.fda.gov/media/70858/download. Accessed 29 Oct 2019.
Reder S, Riffelmann M, Becker C, Wirsing von König CH. Measuring immunoglobulin G antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin Vaccine Immunol. 2008;15:744–9. https://doi.org/10.1128/CVI.00225-07.
Manghi MA, Pasetti MF, Brero ML, Deluchi S, di Paola G, Mathet V, et al. Development of an ELISA for measuring the activity of tetanus toxoid in vaccines and comparison with the toxin neutralization test in mice. J Immunol Methods. 1994;168:17–24. https://doi.org/10.1016/0022-1759(94)90204-6.
Ahnert-Hilger G, Bizzini B, Goretzki K, Müller H, Völckers C, Habermann E. Monoclonal antibodies against tetanus toxin and toxoid. Med Microbiol Immunol. 1983;172:123–35. https://doi.org/10.1007/BF02124513.
Volk WA, Bizzini B, Snyder RM, Bernhard E, Wagner RR. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect Immun. 1984;45:604–9. https://doi.org/10.1128/iai.45.3.604-609.1984.
Kaittanis C, Santra S, Santiesteban OJ, Henderson TJ, Perez JM. The assembly state between magnetic nanosensors and their targets orchestrates their magnetic relaxation response. J Am Chem Soc. 2011;133:3668–76. https://doi.org/10.1021/ja1109584.
Blümich B. Low-field and benchtop NMR. J Magn Reson. 2019;306:27–35. https://doi.org/10.1016/j.jmr.2019.07.030.
Huber S, Min C, Staat C, Oh J, Castro CM, Haase A, et al. Multichannel digital heteronuclear magnetic resonance biosensor. Biosens Bioelectron. 2019;126:240–8. https://doi.org/10.1016/j.bios.2018.10.052.
Funding
This work was supported by the Russian Science Foundation (Grant No. 17-15-01116).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Research was approved by the Review Board of the Institute of Ecology and Genetics of Microorganisms UB RAS (IRB00010009).
Informed consent
Written informed consent was obtained from volunteers.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 45 kb)
Rights and permissions
About this article
Cite this article
Khramtsov, P., Kropaneva, M., Bochkova, M. et al. Nuclear magnetic resonance immunoassay of tetanus antibodies based on the displacement of magnetic nanoparticles. Anal Bioanal Chem 413, 1461–1471 (2021). https://doi.org/10.1007/s00216-020-03112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03112-7