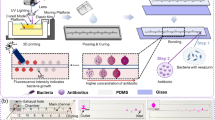
Abstract
Microbial resistance to currently available antibiotics poses a great threat in the global fight against infections. An important step in determining bacterial antibiotic resistance can be selective DNA sequence capture and fluorescence labeling. In this paper, we demonstrate the fabrication of simple, robust, inexpensive microfluidic devices for DNA capture and fluorescence detection of a model antibiotic resistance gene sequence. We laser micromachined polymethyl methacrylate microchannels and enclosed them using pressure-sensitive adhesive tapes. We then formed porous polymer monoliths with DNA capture probes in these microchannels and used them for sequence-specific capture, fluorescent labeling, and laser-induced fluorescence detection of picomolar (pM) concentrations of synthetic and plasmid antibiotic resistance gene targets. The relative fluorescence for the elution peaks increased with loaded target DNA concentration. We observed higher fluorescence signal and percent recovery for synthetic target DNA compared to plasmid DNA at the same loaded target concentration. A non-target gene was used for control experiments and produced < 3% capture relative to the same concentration of target. The full analysis process including device fabrication was completed in less than 90 min with a limit of detection of 30 pM. The simplicity of device fabrication and good DNA capture selectivity demonstrated herein have potential for application with processes for bacterial plasmid DNA extraction and single-particle counting to facilitate determination of antibiotic susceptibility.

Graphical abstract




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (ATW), upon reasonable request.
Change history
07 December 2020
Springer Nature’s version of this paper was updated to remove the “?]” which occured in three places in the published PDF.
References
Belkum AV, Burnham CD, Rossen JWA, Mallard F, Rochas O, Dunne WM Jr. Innovative and rapid antimicrobial susceptibility testing systems. Nat Rev Microbiol. 2020;18:299–311.
Tannert A, Ramoji A, Neugebauer U, Popp J. Photonic monitoring of treatment during infection and sepsis: development of new detection strategies and potential clinical applications. Anal Bioanal Chem. 2018;410:773–90.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
Balouiri M, Sadiki M, Koraichi IS. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–9.
Benkova M, Soukup O, Marek J. Antimicrobial susceptibility testing: currently used methods and devices and the near future in clinical practice. J Appl Microbiol. 2020;129:806–22.
Broeren MAC, Maas Y, Retera E, Arents NLA. Antimicrobial susceptibility testing in 90 min by bacterial cell count monitoring. Clin Microbiol Infect. 2013;19:286–91.
Li Y, Fan P, Zhou S, Zhang L. Loop-mediated isothermal amplification (LAMP): a novel rapid detection platform for pathogens. Microb Pathog. 2017;107:54–61.
Dapprich J, Ferriola D, Mackiewicz K, Clark PM, Rappaport E, D’Arcy M, et al. The next generation of target capture technologies - large DNA fragment enrichment and sequencing determines regional genomic variation of high complexity. BMC Genomics. 2016;17:486.
Dong T, Wang GA, Li F. Shaping up field-deployable nucleic acid testing using microfluidic paper-based analytical devices. Anal Bioanal Chem. 2019;411:4401–14.
Foudeh AM, Didar TF, Veres T, Tabrizian M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12:3249–66.
Pagaduan JV, Sahore V, Woolley AT. Applications of microfluidics and microchip electrophoresis for potential clinical biomarker analysis. Anal Bioanal Chem. 2015;407:6911–22.
Nielsen JB, Hanson RL, Almughamsi HA, Pang C, Fish TR, Woolley AT. Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem. 2020;92:150–68.
Ahrberg CD, Manz A, Chung BG. Polymerase chain reaction in microfluidic devices. Lab Chip. 2016;16:3866–84.
Meena GG, Hanson RL, Wood RL, Brown OT, Stott MA, Robison RA, et al. 3× multiplexed detection of antibiotic resistant plasmids with single molecule sensitivity. Lab Chip. 2020;20:3763–71.
Tan SJ, Phan H, Gerry BM, Kuhn A, Hong LZ, Ong YM, et al. A microfluidic device for preparing next generation DNA sequencing libraries and for automating other laboratory protocols that require one or more column chromatography steps. PLoS One. 2013;8:e64084.
Noviana E, Jain S, Hofstetter J, Geiss BJ, Dandy DS, Henry CS. Paper-based nuclease protection assay with on-chip sample pretreatment for point-of-need nucleic acid detection. Anal Bioanal Chem. 2020;412:3051–61.
Shokoufi N, Asbaghi BAN, Abbasi-Ahd A. Microfluidic chip-photothermal lens microscopy for DNA hybridization assay using gold nanoparticles. Anal Bioanal Chem. 2019;411:6119–28.
Wang J, Morabito K, Tang JX, Tripathi A. Microfluidic platform for isolating nucleic acid targets using sequence specific hybridization. Biomicrofluidics. 2013;7:044107.
Knob R, Hanson RL, Tateoka OB, Wood RL, Guerrero-Arguero I, Robison RA, et al. Sequence-specific sepsis-related DNA capture and fluorescent labeling in monoliths prepared by single-step photopolymerization in microfluidic devices. J Chromatogr A. 2018;1562:12–8.
Hong TF, Ju WJ, Wu MC, Tai CH, Tsai CH, Fu LM. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid Nanofluid. 2010;9:1125–33.
Yang H, Gijs MAM. Micro-optics for microfluidic analytical applications. Chem Soc Rev. 2018;47:1391–458.
Liga A, Morton JAS, Kersaudy-Kerhoas M. Safe and cost-effective rapid-prototyping of multilayer PMMA microfluidic devices. Microfluid Nanofluid. 2016;20:164.
Matellan C, Hernández AER. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci Rep. 2018;8:6971.
Sivakumar R, Lee NY. Microfluidic device fabrication mediated by surface chemical bonding. Analyst. 2020;145:4096–110.
Wang X, Zhang L, Chen G. Hot embossing and thermal bonding of poly(methyl methacrylate) microfluidic chips using positive temperature coefficient ceramic heater. Anal Bioanal Chem. 2011;401:2657–65.
Bazaz SR, Rouhi O, Raoufi MA, Ejeian F, Asadnia M, Jin D, et al. 3D printing of inertial microfluidic devices. Sci Rep. 2020;10:5929.
Kinahan DJ, Early PL, Vembadi A, MacNamara E, Kilcawley NA, Glennon T, et al. Xurography actuated valving for centrifugal flow control. Lab Chip. 2016;16:3454–9.
Serra M, Pereiro I, Yamada A, Viovy JL, Desroix S, Ferraro D. A simple and low-cost chip bonding solution for high pressure, high temperature and biological applications. Lab Chip. 2017;17:629–34.
Tsao C, Syu W. Bonding of thermoplastic microfluidics by using dry adhesive tape. RSC Adv. 2020;10:30289–96.
Knob R, Nelson DB, Robison RA, Woolley AT. Sequence-specific DNA solid-phase extraction in an on-chip monolith: towards detection of antibiotic resistance genes. J Chromatogr A. 2017;1523:309–15.
Rogers CI, Nordin GP, Woolley AT. Optimization of a single-monomer formulation of polyethylene glycol diacrylate as a nonadsorptive polymeric material for microfluidics. Anal Chem. 2011;83:6418–25.
Buchanan CM, Wood RL, Hoj TR, Alizadeh M, Bledsoe CG, Wood ME, et al. Rapid separation of very low concentrations of bacteria from blood. J Microbiol Methods. 2017;139:48–53.
Ogilvie IRG, Sieben VJ, Floquet CFA, Zmijan R, Mowlem MC, Morgan H. Reduction of surface roughness for optical quality microfluidic devices in PMMA and COC. J Micromech Microeng. 2010;20:065016.
Funding
This research was funded by the U.S. National Institutes of Health (grant R01 AI116989).
Author information
Authors and Affiliations
Contributions
Not applicable
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants and/or animals
None
Ethics approval
Not applicable
Informed consent
Not applicable
Consent for publication
The authors give their consent for the article to be published in Anal. Bioanal. Chem.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 373 kb)
Rights and permissions
About this article
Cite this article
Akuoko, Y., Hanson, R.L., Harris, D.H. et al. Rapid and simple pressure-sensitive adhesive microdevice fabrication for sequence-specific capture and fluorescence detection of sepsis-related bacterial plasmid gene sequences. Anal Bioanal Chem 413, 1017–1025 (2021). https://doi.org/10.1007/s00216-020-03060-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03060-2