Abstract
Detecting the administration of naturally occurring but synthetically derived steroids (e.g., testosterone) in routine doping controls is particularly laborious and time-consuming. Carbon isotope signatures determined by isotope ratio mass spectrometry (IRMS) have been established as the method of choice to generate confirmatory evidence in case of suspicious or atypical findings in steroid profile analyses; however, IRMS measurements require sophisticated sample preparation methods employing up to two high-performance liquid chromatography (HPLC) purification steps. Here, an alternative sample preparation approach is presented. Immunoaffinity chromatography (IAC) was employed to reduce the batch analysis time by omitting the time-consuming HPLC purification steps, while pre- and post-IAC sample handling followed published protocols. IAC exploits specific antibody-immunogen interactions, and the option of combining three immunoaffinity gels containing specific antibodies for testosterone, pregnanediol, and 11-ketoetiocholanolone into a multi-immunoaffinity sample preparation approach was assessed. Due to cross reactivities, also etiocholanolone, androsterone, 5β-androstanediol, and 5α-androstanediol were co-extracted and included in the testing protocol. The method was validated by determining precision, recovery, and carry over, and performing linear mixing models. IAC was found to be applicable to the determination of carbon isotope ratios in doping controls and the approach allowed for an accelerated sample preparation.

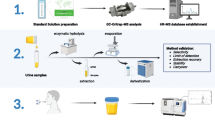
Graphical abstract


Similar content being viewed by others
References
The 2019 Prohibited List. World Anti-Doping Agency. 2019. https://www.wada-ama.org/sites/default/files/wada_2019_english_prohibited_list.pdf. Accessed 15 May 2019.
2017 Anti-doping testing figures. World Anti-Doping Agency. 2018. https://www.wada-ama.org/sites/default/files/resources/files/2017_anti-doping_testing_figures_en_0.pdf. Accessed 15 May 2019.
Mareck U, Geyer H, Opfermann G, Thevis M, Schänzer W. Factors influencing the steroid profile in doping control analysis. J Mass Spectrom. 2008;43(7):877–91.
TD2019-IRMS. World Anti-Doping Agency. 2019. https://www.wada-ama.org/sites/default/files/td2019irms_final_eng_clean.pdf. Accessed 18 March 2019.
TD2018-EAAS. World Anti-Doping Agency. 2018. https://www.wada-ama.org/sites/default/files/resources/files/td2018eaas_final_eng.pdf. Accessed 15 May 2019.
Piper T, Emery C, Saugy M. Recent developments in the use of isotope ratio mass spectrometry in sports drug testing. Anal Bioanal Chem. 2011;401(2):433–47.
de la Torre X, González JC, Pichini S, Pascual JA, Segura J. 13C/12C isotope ratio MS analysis of testosterone, in chemicals and pharmaceutical preparations. J Pharm Biomed Anal. 2001;24(4):645–50.
Piper T, Thevis M. Applications of isotope ratio mass spectrometry in sports drug testing accounting for isotope fractionation in analysis of biological samples. Methods Enzymol. 2017;596(1557–7988):403–32.
Fitzgerald J, Leonard P, Darcy E, Sharma S, O'Kennedy R. Immunoaffinity chromatography: concepts and applications. Methods Mol Biol. 2017;1485:27–51.
Hage DS, Anguizola JA, Li R, Matsuda R, Papastavros E, Pfaunmiller E, et al. Chapter 12 - Affinity chromatography. In: Fanali S, Haddad PR, Poole CF, Riekkola M-L, editors. Liquid chromatography. 2nd ed: Elsevier; 2017. p. 319–41.
Chen HX, Deng QP, Zhang LW, Zhang XX. Quantification of testosterone and epitestosterone in biological samples by capillary electrophoresis with immunoaffinity extraction. Talanta. 2009;78(2):464–70.
Chiesa L, Nobile M, Panseri S, Sgoifo Rossi CA, Pavlovic R, Arioli F. Detection of boldenone, its conjugates and androstadienedione, as well as five corticosteroids in bovine bile through a unique immunoaffinity column clean-up and two validated liquid chromatography-tandem mass spectrometry analyses. Anal Chim Acta. 2014;852:137–45.
Chiesa L, Pavlovic R, Dusi G, Pasquale E, Casati A, Panseri S, et al. Determination of alpha- and beta-boldenone sulfate, glucuronide and free forms, and androstadienedione in bovine urine using immunoaffinity columns clean-up and liquid chromatography tandem mass spectrometry analysis. Talanta. 2015;131:163–9.
Gasparini M, Curatolo M, Assini W, Bozzoni E, Tognoli N, Dusi G. Confirmatory method for the determination of nandrolone and trenbolone in urine samples using immunoaffinity cleanup and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2009;1216(46):8059–66.
Machnik M, Delahaut P, Horning S, Schänzer W, editors. Preparation of immuno affinity columns. Recent advances in doping analysis. 1998; Cologne: Sport u. Buch Strauß.
McEvoy JD, McCaughey WJ, Cooper J, Kennedy DG, McCartan BM. Nortestosterone is not a naturally occurring compound in male cattle. Vet Q. 1999;21(1):8–15.
Qi XH, Zhang LW, Zhang XX. Simultaneous determination of nandrolone, testosterone, and methyltestosterone by multi-immunoaffinity column and capillary electrophoresis. Electrophoresis. 2008;29(16):3398–405.
Schänzer W, Delahaut P, Völker E, Donike M, editors. Immunoaffinity chromatography in isolation of anabolic steroids. Recent advances in doping analysis. 1993; Cologne: Sport u. Buch Strauß.
van Ginkel LA, Stephany RW, van Rossum HJ, van Blitterswijk H, Zoontjes PW, Hooijschuur RCM, et al. Effective monitoring of residues of nortestosterone and its major metabolite in bovine urine and bile. J Chromatogr. 1989;489:95–104.
van Ginkel LA, van Blitterswijk H, Zoontjes PW, van den Bosch D, Stephany RW. Assay for trenbolone and its metabolite 17 alpha-trenbolone in bovine urine based on immunoaffinity chromatographic clean-up and off-line high-performance liquid chromatography-thin-layer chromatography. J Chromatogr. 1988;445:385–92.
Horning S, Geyer H, Machnik M, Schänzer W, Hilkert A, Oeßelmann J, editors. Detection of exogenous testosterone by 13C/12C analysis. Recent advances in doping analysis. 1997; Cologne: Sport u. Buch Strauß.
Aguilera R, Becchi M, Grenot C, Casabianca H, Hatton CK. Detection of testosterone misuse: comparison of two chromatographic sample preparation methods for gas chromatographic-combustion/isotope ratio mass spectrometric analysis. J Chromatogr B Biomed Appl. 1996;687(1):43–53.
Desroches MC, Mathurin JC, Richard Y, Delahaut P, de Ceaurriz J. Urinary 19-norandrosterone purification by immunoaffinity chromatography: application to gas chromatography/combustion/isotope ratio mass spectrometric analysis. Rapid Commun Mass Spectrom. 2002;16(5):370–4.
Delahaut P, Jacquemin P, Colemonts Y, Dubois M, De Graeve J, Deluyker H. Quantitative determination of several synthetic corticosteriods by gas chromatography-mass spectrometry after purification by immunoaffinity chromatography. J Chromatogr B Biomed Sci Appl. 1997;696(2):203–15.
Dubois M, Taillieu X, Colemonts Y, Lansival B, De Graeve J, Delahaut P. GC-MS determination of anabolic steroids after multi-immunoaffinity purification. Analyst. 1998;123(12):2611–6.
van Ginkel LA, Stephany RW, van Rossum HJ, Steinbuch HM, Zomer G, Van de Heeft E, et al. Multi-immunoaffinity chromatography: a simple and highly selective clean-up method for multi-anabolic residue analysis of meat. J Chromatogr. 1989;489(1):111–20.
Jedziniak P, Panasiuk L, Pietruszka K, Posyniak A. Multiple mycotoxins analysis in animal feed with LC-MS/MS: comparison of extract dilution and immunoaffinity clean-up. J Sep Sci. 2019;42(6):1240–7.
Kaymak T, Koca E, Atak M, Sarikaya E, Stroka J. Determination of aflatoxins and ochratoxin a in traditional Turkish cereal-based fermented food by multi-affinity column cleanup and LC fluorescence detection: single-laboratory validation. J AOAC Int. 2018;102:156–63.
Zhang Y, Pei F, Fang Y, Li P, Zhao Y, Shen F, et al. Comparison of concentration and health risks of 9 Fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC. Food Chem. 2019;275:763–9.
Piper T, Mareck U, Geyer H, Flenker U, Thevis M, Platen P, et al. Determination of 13C/12C ratios of endogenous urinary steroids: method validation, reference population and application to doping control purposes. Rapid Commun Mass Spectrom. 2008;22(14):2161–75.
Saudan C, Emery C, Marclay F, Strahm E, Mangin P, Saugy M. Validation and performance comparison of two carbon isotope ratio methods to control the misuse of androgens in humans. J Chromatogr B. 2009;877(23):2321–9.
Putz M, Piper T, Casilli A, Radler de Aquino Neto F, Pigozzo F, Thevis M. Development and validation of a multidimensional gas chromatography/combustion/isotope ratio mass spectrometry-based test method for analyzing urinary steroids in doping controls. Anal Chim Acta. 2018;1030:105–14.
Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981;27(3):493.
Werner RA, Brand WA. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom. 2001;15(7):501–19.
Docherty G, Jones V, Evershed RP. Practical and theoretical considerations in the gas chromatography/combustion/isotope ratio mass spectrometry delta(13)C analysis of small polyfunctional compounds. Rapid Commun Mass Spectrom. 2001;15(9):730–8.
Piper T, Thevis M, Flenker U, Schänzer W. Determination of the deuterium/hydrogen ratio of endogenous urinary steroids for doping control purposes. Rapid Commun Mass Spectrom. 2009;23(13):1917–26.
Piper T, Flenker U, Mareck U, Schänzer W. 13C/12C ratios of endogenous urinary steroids investigated for doping control purposes. Drug Test Anal. 2009;1(2):65–72.
Piper T, Putz M, Delahaut P, Thevis M. Carbon isotope ratios of endogenous steroids in Belgian blue and Holstein cattle: method development, reference population studies and application to steroid misuse control. Rapid Commun Mass Spectrom. 2017;31(21):1793–802.
de la Torre X, Colamonici C, Curcio D, Molaioni F, Botre F. A comprehensive procedure based on gas chromatography-isotope ratio mass spectrometry following high performance liquid chromatography purification for the analysis of underivatized testosterone and its analogues in human urine. Anal Chim Acta. 2012;756:23–9.
Piper T, Emery C, Thomas A, Saugy M, Thevis M. Combination of carbon isotope ratio with hydrogen isotope ratio determinations in sports drug testing. Anal Bioanal Chem. 2013;405(16):5455–66.
Keeling CD. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Acta. 1958;13(4):322–34.
de la Torre X, Colamonici C, Curcio D, Molaioni F, Pizzardi M, Botre F. A simplified procedure for GC/C/IRMS analysis of underivatized 19-norandrosterone in urine following HPLC purification. Steroids. 2011;76(5):471–7.
Mareck U, Thevis M, Guddat S, Gotzmann A, Bredehöft M, Geyer H, et al., editors. Comprehensive sample preparation for anabolic steroids, glucocorticosteroids, beta-receptor blocking agents, selected anabolic androgenic steroids and Buprenorphine in human urine. Recent advances in doping analysis. 2004; Cologne: Sport u. Buch Strauß.
Acknowledgements
This project was supported by the Manfred-Donike Institute for Dope Analysis (Cologne, Germany) and the World Anti-Doping Agency (grant #16A17MT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies involving human participants were approved by the Ethics Committee of the German Sport University Cologne according to the declaration of Helsinki. Informed consent was obtained from all volunteers. Care and use of animals were in compliance with the directive 2010/63/EU on the protection of animals used for scientific purposes. The animal studies were approved by the Group’s CER Ethics Committee (CE/Sante/E/001).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Putz, M., Piper, T., Dubois, M. et al. Analysis of endogenous steroids in urine by means of multi-immunoaffinity chromatography and isotope ratio mass spectrometry for sports drug testing. Anal Bioanal Chem 411, 7563–7571 (2019). https://doi.org/10.1007/s00216-019-02169-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02169-3