Abstract
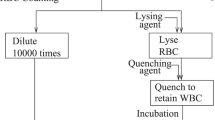
Blood counting is one of the most commonly ordered clinical assays, and is often part of the basis for initial diagnosis and screening for disease. While substantial prior research has shown the ability of portable instruments to accurately produce blood counts through image- or flow-based cytometry, these methods require complex sample preparation using either costly commercial imaging chambers or complicated reagents. To address these issues, in this paper we developed a method to prepare trace volumes of whole blood aimed at portable blood counting. The strategy is based on pre-storing dry-form reagents and fabricating a specifically designed cell counter. In order to obtain total cell counts for red blood cells, platelets, and 3-part differentials of white blood cells, two parallel counting chambers with different depths are made from cost- and environmentally friendly materials using soft lithography. As little as 1 μl of whole blood is prepared with pre-stored reagents in centrifuge vials, whereas red blood cells are sphered and white blood cells are stained at the same time. Driven by the capillary force, prepared blood samples enter the hydrophilic chambers automatically. Monolayers of cells are formed when the blood dilution factors and the chamber depths are co-optimized. Combined with our previous custom-built instrument and automated analysis algorithm, the sample preparation strategy allows producing counting results with excellent agreement to a gold-standard clinical hematology instrument. The success of this preparation method may further advance applications of our technology for global use in low-resource settings where central hematology laboratories are not accessible.

Graphical Abstract






Similar content being viewed by others
References
Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923–36.
Verbrugge SE, Huisman A. Verification and standardization of blood cell counters for routine clinical laboratory tests. Clin Lab Med. 2015;35(1):183–96.
Chabot-Richards DS, George TI. White blood cell counts reference methodology. Clin Lab Med. 2014;35(1):11–24.
Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9(9):e1001306.
Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect. 2010;16(8):1062–9.
Peters DH, Garg A, Bloom G, Walker DG, Brieger WR, Rahman MH. Poverty and access to health care in developing countries. Ann N Y Acad Sci. 2008;1136(1):161–70.
Boppart SA, Richards-Kortum R. Point-of-care and point-of-procedure optical imaging technologies for primary care and global health. Sci Transl Med. 2014;6(253):253rv2.
Browne AW, Ramasamy L, Cripe TP, Ahn CH. A lab-on-a-chip for rapid blood separation and quantification of hematocrit and serum analytes. Lab Chip. 2011;11(14):2440–6.
Vercruysse D, Dusa A, Stahl R, Vanmeerbeeck G, Wijs KD, Liu C, et al. Three-part differential of unlabeled leukocytes with a compact lens-free imaging flow cytometer. Lab Chip. 2015;15(4):1123–32.
Shi W, Guo L, Kasdan H, Tai YC. Four-part leukocyte differential count based on sheathless microflow cytometer and fluorescent dye assay. Lab Chip. 2013;13(7):1257–65.
Berkel CV, Gwyer JD, Deane S, Green N, Holloway J, Hollis V, et al. Integrated systems for rapid point of care (PoC) blood cell analysis. Lab Chip. 2011;11(7):1249–55.
Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal Chem. 2011;83(17):6641–7.
Zhu H, Sencan I, Wong J, Dimitrov S, Tseng D, Nagashima K, et al. Cost-effective and rapid blood analysis on a cell-phone. Lab Chip. 2013;13(7):1282–8.
Ben-Yosef Y, Marom B, Hirshberg G, D'Souza C, Larsson A, Bransky A. The HemoScreen, a novel haematology analyser for the point of care. J Clin Pathol. 2016;69(8):720–5.
Leshansky AM, Bransky A, Korin N, Dinnar U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys Rev Lett. 2007;98(23):234501.
Del Giudice F, Romeo G, D’Avino G, Greco F, Netti P, Maffettone PL. Particle alignment in a viscoelastic liquid flowing in a square-shaped microchannel. Lab Chip. 2013;13(21):4263–71.
D’Avino G, Romeo G, Villone MM, Greco F, Netti PA, Maffettone PL. Single line particle focusing induced by viscoelasticity of the suspending liquid: theory, experiments and simulations to design a micropipe flow-focuser. Lab Chip. 2012;12(9):1638–45.
Song Y, Huang Y, Liu X, Zhang X, Ferrari M, Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014;32(3):132–9.
Pacaud D, Lemay J, Guay P, Buithieu M, Yale J. Assessment of blood volumes obtained by capillary punctures in older children and adolescents with diabetes. Pediatr Res. 1996;39:95.
Wong ECC. Hematology analyzers: special considerations for pediatric patients. Clin Lab Med. 2015;35:165–81.
Smith ZJ, Gao T, Chu K, Lane SM, Matthews DL, Dwyre DM, et al. Single-step preparation and image-based counting of minute volumes of human blood. Lab Chip. 2014;14(16):3029–36.
Smith ZJ, Gao T, Lane SM, Wachmann-Hogiu S, Dwyre DM, Heifetz L, et al. Portable Blood Count Monitor, U.S. Patent No. 20,140,273,064. Washington, DC: U.S. Patent and Trademark Office; 2014.
Gao T, Smith ZJ, Lin T, Holt DC, Lane SM, Matthews DL, et al. Smart and fast blood counting of trace volumes of body fluids from various mammalian species using a compact, custom-built microscope cytometer. Anal Chem. 2015;87(23):11854–62.
Xie D, Xie Y, Liu P, Tong L, Hu C, Shao P, et al. Performance of a cost-effective and automated blood counting system for resource-limited settings operated by trained and untrained users. J Biophotonics. 2018;11(2):e201700030.
Kim YR, Ornstein L. Isovolurnetric sphering of erythrocytes for more accurate and precise cell volume measurement by flow cytometry. Cytometry. 1983;3(6):419–27.
Ban T, Kasatani K, Kawasaki M, Sato H. Fluorescence decay of the acridine orange-sodium dodecyl sulfate system: formation of dye-rich induced micelles in the premicellar region. Photochem Photobiol. 1983;37(2):131–9.
Moulik SP, Ghosh S, Das AR. Interaction of acridine orange monohydrochloride dye with sodiumdodeeylsulfate (SDS), cetyltrimethylammoniumbromide (CTAB) and p-tert-octylphenoxypolyoxy ethanol (Triton X 100) surfactants. Colloid Polym Sci. 1979;257(6):645–55.
Gerola AP, Costa PF, Quina FH, Fiedler HD, Nome F. Zwitterionic surfactants in ion binding and catalysis. Curr Opin Colloid Interface Sci. 2017;32:39–47.
Nabity MB, Harr KE, Camus MS, Flatland B, Vap LM. ASVCP guidelines: allowable total error hematology. Vet Clin Pathol. 2018;47(1):9–21.
Buttarello M, Plebani M. Automated blood cell counts. Am J Clin Pathol. 2008;130(1):104–15.
Funding
This work is supported by the National Natural Science Foundation of China (21605054), Central China Normal University (CCNU) new PI start-up fund (210-31102), and self-determined research funds of CCNU (234-20205016002, 234-20205170355) from the colleges’ basic research and operation of Ministry of Education (MOE). TG acknowledges support from the Program of Introducing Talents of Discipline to University of China (111 program, B17019), and the CCNU Program of Innovation and Entrepreneurship for college students (220-20205180419). ZJS acknowledges support from the 1000 Young Talents Global Recruitment Plan, and from the Ministry of Science and Technology of the People’s Republic of China (2016YFA020130).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 849 kb)
Rights and permissions
About this article
Cite this article
Li, X., Deng, Q., Liu, H. et al. A smart preparation strategy for point-of-care cellular counting of trace volumes of human blood. Anal Bioanal Chem 411, 2767–2780 (2019). https://doi.org/10.1007/s00216-019-01738-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01738-w