Abstract
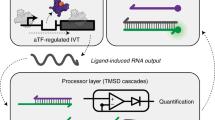
The emerging field of RNA nanotechnology harnesses the versatility of RNA molecules to generate nature-inspired systems with programmable structure and functionality. Such methodology has therefore gained appeal in the fields of biosensing and diagnostics, where specific molecular recognition and advanced input/output processing are demanded. The use of RNA modules and components allows for achieving diversity in structure and function, for processing information with molecular precision, and for programming dynamic operations on the grounds of predictable non-covalent interactions. When RNA nanotechnology meets bioanalytical chemistry, sensing of target molecules can be performed by harnessing programmable interactions of RNA modules, advanced field-ready biosensors can be manufactured by interfacing RNA-based devices with supporting portable platforms, and RNA sensors can be engineered to be genetically encoded allowing for real-time imaging of biomolecules in living cells. In this article, we report recent advances in RNA-based sensing technologies and discuss current trends in RNA nanotechnology-enabled biomedical diagnostics. In particular, we describe programmable sensors that leverage modular designs comprising dynamic aptamer-based units, synthetic RNA nanodevices able to perform target-responsive regulation of gene expression, and paper-based sensors incorporating artificial RNA networks.

ᅟ




Similar content being viewed by others
References
Engelhart AE. RNA imaging: a tale of two G-quadruplexes. Nat Chem Biol. 2017;13(11):1140–1.
Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5(12):833–42.
Grabow WW, Jaeger L. RNA self-assembly and RNA nanotechnology. Acc Chem Res. 2014;47(6):1871–80.
Jasinski D, Haque F, Binzel DW, Guo P. Advancement of the emerging field of RNA nanotechnology. ACS Nano. 2017;11(2):1142–64.
Chappell J, Watters KE, Takahashi MK, Lucks JB. A renaissance in RNA synthetic biology: new mechanisms, applications and tools for the future. Curr Opin Chem Biol. 2015;28:47–56.
Slomovic S, Pardee K, Collins JJ. Synthetic biology devices for in vitro and in vivo diagnostics. Proc Natl Acad Sci U S A. 2015;112(47):14429–35.
Bailey RC. Grand Challenge Commentary: Informative diagnostics for personalized medicine. Nat Chem Biol. 2010;6(12):857–9.
Bouhedda F, Autour A, Ryckelynck M. Light-up RNA aptamers and their cognate fluorogens: from their development to their applications. Int J Mol Sci. 2018;19(1):E44.
Neubacher S, Hennig S. RNA structure and cellular applications of fluorescent light-up aptamers. Angew Chem Int Ed. 2018. https://doi.org/10.1002/anie.201806482.
Ouellet J. RNA Fluorescence with light-up aptamers. Front Chem. 2016;4:29.
Szeto K, Latulippe DR, Ozer A, Pagano JM, White BS, Shalloway D, et al. RAPID-SELEX for RNA aptamers. PLoS One. 2013;8(12):e82667.
Gotrik M, Sekhon G, Saurabh S, Nakamoto M, Eisenstein M, Soh HT. Direct selection of fluorescence-enhancing RNA aptamers. J Am Chem Soc. 2018;140(10):3583–91.
Xu W, Lu Y. Label-free fluorescent aptamer sensor based on regulation of malachite green fluorescence. Anal Chem. 2010;82(2):574–8.
Bang GS, Cho S, Lee N, Lee B-R, Kim J-H, Kim B-G. Rational design of modular allosteric aptamer sensor for label-free protein detection. Biosens Bioelectron. 2013;39(1):44–50.
Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science. 2011;333(6042):642–6.
Filonov GS, Moon JD, Svensen N, Jaffrey SR. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J Am Chem Soc. 2014;136(46):16299–308.
Dolgosheina EV, Jeng SCY, Panchapakesan SSS, Cojocaru R, Chen PSK, Wilson PD, et al. RNA Mango aptamer-fluorophore: a bright, high-affinity complex for RNA labeling and tracking. ACS Chem Biol. 2014;9(10):2412–20.
You M, Litke JL, Jaffrey SR. Imaging metabolite dynamics in living cells using a Spinach-based riboswitch. Proc Natl Acad Sci. 2015;112(21):E2756.
Ying Z-M, Wu Z, Tu B, Tan W, Jiang J-H. Genetically encoded fluorescent RNA sensor for ratiometric imaging of microRNA in living tumor cells. J Am Chem Soc. 2017;139(29):9779–82.
Jepsen MDE, Sparvath SM, Nielsen TB, Langvad AH, Grossi G, Gothelf KV, et al. Development of a genetically encodable FRET system using fluorescent RNA aptamers. Nat Commun. 2018;9(1):18.
Warner KD, Chen MC, Song W, Strack RL, Thorn A, Jaffrey SR, et al. Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat Struct Mol Biol. 2014;21(8):658–63.
Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335(6073):1194.
Park MH, Igarashi K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol Ther. 2013;21(1):1–9.
Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Metabolite profiling: from diagnostics to systems biology. Nat Rev Mol Cell Biol. 2004;5(9):763–9.
Aw SS, Tang MX, Teo YN, Cohen SM. A conformation-induced fluorescence method for microRNA detection. Nucleic Acids Res. 2016;44(10):e92.
Huang K, Doyle F, Wurz ZE, Tenenbaum SA, Hammond RK, Caplan JL, et al. FASTmiR: an RNA-based sensor for in vitro quantification and live-cell localization of small RNAs. Nucleic Acids Res. 2017;45(14):e130.
Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2008;13(1):39–53.
Autour A, Jeng SCY, Cawte DA, Abdolahzadeh A, Galli A, Panchapakesan SSS, et al. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat Commun. 2018;9(1):656.
Wang Z, Luo Y, Xie X, Hu X, Song H, Zhao Y, et al. In situ spatial complementation of aptamer-mediated recognition enables live-cell imaging of native RNA transcripts in real time. Angew Chem Int Ed. 2018;57(4):972–6.
Bertucci A, Porchetta A, Ricci F. Antibody-templated assembly of an RNA mimic of green fluorescent protein. Anal Chem. 2018;90(2):1049–53.
Karunanayake Mudiyanselage APKK, Yu Q, Leon-Duque MA, Zhao B, Wu R, You M. Genetically encoded catalytic hairpin assembly for sensitive RNA imaging in live cells. J Am Chem Soc. 2018;140(28):8739–45.
Michnick SW, Ear PH, Manderson EN, Remy I, Stefan E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat Rev Drug Discov. 2007;6(7):569–82.
Zhang H, Li F, Dever B, Wang C, Li X-F, Le XC. Assembling DNA through affinity binding to achieve ultrasensitive protein detection. Angew Chem Int Ed. 2013;52(41):10698–705.
Kolpashchikov DM. Binary malachite green aptamer for fluorescent detection of nucleic acids. J Am Chem Soc. 2005;127(36):12442–3.
Kikuchi N, Kolpashchikov DM. Split Spinach aptamer for highly selective recognition of DNA and RNA at ambient temperatures. Chembiochem. 2016;17(17):1589–92.
Kikuchi N, Kolpashchikov DM. A universal split spinach aptamer (USSA) for nucleic acid analysis and DNA computation. Chem Commun. 2017;53(36):4977–80.
Rogers TA, Andrews GE, Jaeger L, Grabow WW. Fluorescent monitoring of RNA assembly and processing using the split-Spinach aptamer. ACS Synth Biol. 2015;4(2):162–6.
Alam KK, Tawiah KD, Lichte MF, Porciani D, Burke DH. A fluorescent split aptamer for visualizing RNA-RNA assembly in vivo. ACS Synth Biol. 2017;6(9):1710–21.
Xie M, Fussenegger M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat Rev Mol Cell Biol. 2018;19(8):507–25.
Masubuchi T, Endo M, Iizuka R, Iguchi A, Yoon DH, Sekiguchi T, et al. Construction of integrated gene logic-chip. Nat Nanotechnol. 2018;13(10):933–40.
Nielsen AAK, Der BS, Shin J, Vaidyanathan P, Paralanov V, Strychalski EA, et al. Genetic circuit design automation. Science. 2016;352(6281):7341.
McKeague M, Wong RS, Smolke CD. Opportunities in the design and application of RNA for gene expression control. Nucleic Acids Res. 2016;44(7):2987–99.
Etzel M, Mörl M. Synthetic riboswitches: from plug and pray toward plug and play. Biochemistry. 2017;56(9):1181–98.
Chen YY, Jensen MC, Smolke CD. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc Natl Acad Sci U S A. 2010;107(19):8531–6.
Isaacs FJ, Dwyer DJ, Ding C, Pervouchine DD, Cantor CR, Collins JJ. Engineered riboregulators enable post-transcriptional control of gene expression. Nat Biotechnol. 2004;22(7):841–7.
Bayer TS, Smolke CD. Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat Biotechnol. 2005;23(3):337–43.
Mutalik VK, Qi L, Guimaraes JC, Lucks JB, Arkin AP. Rationally designed families of orthogonal RNA regulators of translation. Nat Chem Biol. 2012;8(5):447–54.
Liu CC, Qi L, Lucks JB, Segall-Shapiro TH, Wang D, Mutalik VK, et al. An adaptor from translational to transcriptional control enables predictable assembly of complex regulation. Nat Methods. 2012;9(11):1088–94.
Isaacs FJ. Synthetic biology: automated design of RNA devices. Nat Chem Biol. 2012;8(5):413–5.
Green AA, Silver PA, Collins JJ, Yin P. Toehold switches: de-novo-designed regulators of gene expression. Cell. 2014;159(4):925–39.
Karig DK. Cell-free synthetic biology for environmental sensing and remediation. Curr Opin Biotechnol. 2017;45:69–75.
Parolo C, Merkoçi A. Paper-based nanobiosensors for diagnostics. Chem Soc Rev. 2013;42:450–7.
Yamada K, Shibata H, Suzuki K, Citterio D. Toward practical application of paper-based microfluidics for medical diagnostics: state-of-the-art and challenges. Lab Chip. 2017;17:1206–49.
Pardee K, Green AA, Ferrante T, Cameron DE, Daleykeyser A, Yin P, et al. Paper-based synthetic gene networks. Cell. 2014;159(4):940–54.
Takahashi MK, Tan X, Dy AJ, Braff D, Akana RT, Furuta Y, et al. A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat Commun. 2018;9:3347.
Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell. 2016;165(5):1255–66.
Funding
A.P. received support from the University of Rome Tor Vergata under the grant “MIRA” no E81I18000200005. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement no 704120 (“MIRNANO”). A.B. is a global Marie Skłodowska-Curie fellow. M.R. and S.R. are supported from a Fondazione Umberto Veronesi “postdoctoral fellowship 2019”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No experiments involving human participants and/or animals have been conducted for this publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry with guest editors Erin Baker, Kerstin Leopold, Francesco Ricci, and Wei Wang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossetti, M., Del Grosso, E., Ranallo, S. et al. Programmable RNA-based systems for sensing and diagnostic applications. Anal Bioanal Chem 411, 4293–4302 (2019). https://doi.org/10.1007/s00216-019-01622-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01622-7