Abstract
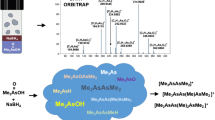
In order to elucidate controversial results emerging in chemical vapor generation (CVG) for trace element determination, we conducted a series of experiments devoted to the identification of intermediates formed by acid hydrolysis of amine-boranes. For the first time, direct analysis in real time coupled with high-resolution mass spectrometry (DART-Orbitrap) was applied for detection of this class of compounds. Mass spectra of both solid amine-boranes and their aqueous solutions (pH ~ 8, no hydrolysis) were acquired for understanding their ionization pathway. Mass spectra of aqueous solutions of t-BuNH2·BH3 and Me2NH·BH3 were acquired under conditions that are employed in CVG (0.017–4.0 mol L−1 HCl, 0.167–0.2 mol L−1 borane reagent). The results disclose a reactivity driven by pH of amine-boranes undergoing hydrolysis. At low acidity, the hydrolysis proceeds according to the currently accepted displacement mechanisms (i.e., R3N·BH3 + H3O+ → R3NH+ + H2OBH3). At higher acidity, N-tert-butyl, cyclotriborazane, and bis(dimethylamino)boronium were identified, for the first time, during the hydrolysis of t-BuNH2·BH3 and Me2NH·BH3, respectively. Formation of these intermediates was ascribed to a hydrolysis pathway starting with the ionization of the amine-borane, (i.e., R3N·BH3 + H3O+ → [(H2O)R3NBH2] + + H2). The new evidence explains the anomalous behavior observed in CVG by amine-borane derivatization, and updates the currently accepted mechanisms for the acid hydrolysis of amine-boranes.

Graphical Abstract









Similar content being viewed by others
References
Dědina J, Tsalev DL. Hydride generation atomic absorption spectrometry. Chichester: Wiley; 1995.
Sturgeon RE, Mester Z. Analytical applications of volatile metal derivatives. Appl Spectrosc. 2002;56:202A–13A.
D’Ulivo A, Loreti V, Onor M, Pitzalis E, Zamboni R. Chemical vapor generation atomic spectrometry using amineboranes and cyanotrihydroborate (III) reagents. Anal Chem. 2003;75:2591–600.
Bramanti E, Lomonte C, Onor M, Zamboni R, D’Ulivo A, Raspi G. Mercury speciation by liquid chromatography coupled with on-line chemical vapour generation and atomic fluorescence spectrometric detection (LC-CVGAFS). Talanta. 2005;66:762–8.
D’Ulivo A, Mester Z, Meija J, Sturgeon RE. Mechanism of generation of volatile hydrides of trace elements by aqueous tetrahydroborate (III). Mass spectrometric studies on reaction products and intermediates. Anal Chem. 2007;79:3008–15.
Welna M, Zyrnicki W. Investigation of simultaneous generation of arsenic, bismuth and antimony hydrides using inductively coupled plasma optical emission spectrometry. Anal Lett. 2011;44:942–53.
Pitzalis E, Onor M, Mascherpa MC, Pacchi G, Mester Z, D’Ulivo A. Chemical generation of arsane and methylarsanes with amine boranes. Potentialities for nonchromatographic speciation of arsenic. Anal Chem. 2014;86:1599–607.
D’Ulivo A, Dědina J, Mester Z, Sturgeon RE, Wang Q, Welz B. Mechanisms of chemical generation of volatile hydrides for trace element determination ( IUPAC Technical Report ). Pure Appl Chem. 2011;83:1283–340.
D’Ulivo A, Baiocchi C, Pitzalis E, Onor M, Zamboni R. Chemical vapor generation for atomic spectrometry. A contribution to the comprehension of reaction mechanisms in the generation of volatile hydrides using borane complexes. Spectrochim Acta B. 2004;59:471–86.
D’Ulivo L, Spiniello R, Onor M, Campanella B, Mester Z, D’Ulivo A. Behavior and kinetic of hydrolysis of amine boranes in acid media employed in chemical vapor generation. Anal Chim Acta. 2018;998:28–36.
Kelly HC, Marchelli FR, Giusto MB. The kinetics and mechanism of solvolysis of amineboranes. Inorg Chem. 1964;3:431–7.
Ryschkewitsch GE. Amine boranes. I. Kinetics of acid hydrolysis of trimethylamine borane. J Am Chem Soc. 1960;82:3290–4.
Kelly HC, Marriott VB. Reexamination of the mechanism of acid-catalyzed amine-borane hydrolysis. The hydrolysis of NH3•BH3. Inorg Chem. 1979;18:2875–8.
D’Ulivo A, Onor M, Pitzalis E. Role of hydroboron intermediates in the mechanism of chemical vapor generation in strongly acidic media. Anal Chem. 2004;76:6342–52.
Cody RB. Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal Chem. 2009;81:1101–7.
Pagliano E, Onor M, McCooeye M, D’Ulivo A, Sturgeon RE, Mester Z. Application of direct analysis in real time to a multiphase chemical system: identification of polymeric arsanes generated by reduction of monomethylarsenate with sodium tetrahydroborate. Int J Mass Spectrom. 2014;371:42–6.
Kreevoy MM, Hutchins JEC. H2BH3as an intermediate in tetrahydridoborate hydrolysis. J Am Chem Soc. 1972;94:6371–6.
Kreevoy MM, Hutchins JEC. Acid-catalyzed hydrolysis and isotope exchange in LiBH3CN. J Am Chem Soc. 1969;91:4329–30.
Stephens FH, Pons V, Tom Baker R. Ammonia–borane: the hydrogen source par excellence? J Chem Soc Dalton Trans. 2007;39:2613–26.
Johnson HC, Hooper TN, Weller AS. The catalytic dehydrocoupling of amine–boranes and phosphine–boranes. Top Organomet Chem. 2015;49:153–220.
Davis RE, Brown AE, Hopmann R, Kibby CL. A rapid and quantitative exchange of the boron hydrogens in thrimethylamine borane with D2O. J Am Chem Soc. 1963;85:497.
Miller NE, Muetterties EL. Chemistry of boranes. X. Borane cations, H2B(base)2+. J Am Chem Soc. 1964;86:1033–8.
Melen RL. Dehydrocoupling routes to element–element bonds catalysed by main group compounds. Chem Soc Rev. 2016;45:775–88.
Jaska CA, Temple K, Lough AJ, Manners I. Transition metal-catalyzed formation of boron-nitrogen bonds: catalytic dehydrocoupling of amine-borane adducts to form aminoboranes and borazines. J Am Chem Soc. 2003;125:9424–34.
Roselló-Merino M, López-Serrano J, Conejero S. Dehydrocoupling reactions of dimethylamine-borane by Pt (II) complexes: a new mechanism involving deprotonation of boronium cations. J Am Chem Soc. 2013;135:10910–3.
Stephens FH, Baker RT, Matus MH, Grant DJ, Dixon DA. Acid initiation of ammonia-borane dehydrogenation for hydrogen storage. Angew Chem Int Ed. 2007;46:746–9.
Pitzalis E, Onor M, Spiniello R, Braz CEM, D’Ulivo A. Mechanism of action of additives in chemical vapor generation of hydrogen selenide: iodide and thiocyanate. Spectrochim Acta B. 2018;145:122–31.
D’Ulivo A. Chemical vapor generation by tetrahydroborate (III) and other borane complexes in aqueous media: a critical discussion of fundamental processes and mechanisms involved in reagent decomposition and hydride formation. Spectrochim Acta B. 2004;59:793–825.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 157 kb)
Rights and permissions
About this article
Cite this article
D’Ulivo, L., Pagliano, E., Onor, M. et al. Application of direct analysis in real time to the study of chemical vapor generation mechanisms: identification of intermediate hydrolysis products of amine-boranes. Anal Bioanal Chem 411, 1569–1578 (2019). https://doi.org/10.1007/s00216-019-01598-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01598-4