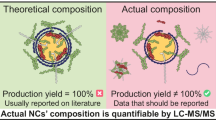
Abstract
A quantitative method of analyzing nanoparticles (NPs) for drug delivery is urgently required by researchers and industry. Therefore, we developed new quantitative analytical methods for biodegradable and amphiphilic NPs consisting of polymeric γ-PGA-Phe [phenylalanine attached to poly(γ-glutamic acid)] molecules. These γ-PGA-Phe NPs were completely dissociated into separate γ-PGA-Phe molecules by adding sodium dodecyl sulfate (SDS). The dissociated NPs were chromatographically separated to analyze parameters such as the γ-PGA-Phe content in the NPs, the impurities present [using reverse-phase (RP) HPLC with an ultraviolet (UV) detector], and the absolute MW [using size-exclusion chromatography (SEC) with refractive index detection (RI) and multiangle light scattering (MALS) detection, i.e., SEC-RI/MALS]. The chromatographic patterns of the NPs were equivalent to those of the component polymer (γ-PGA-Phe), and excellent chromatographic separation for the quantitative evaluation of NPs was achieved. To the best of our knowledge, this is the first report of the quantitative evaluation of NPs in the field of NP-based delivery systems. Furthermore, these methods were applied to optimize and evaluate the NP manufacturing process. The results showed that impurities were effectively removed from the γ-PGA-Phe during the manufacturing process, so the purity of the final γ-PGA-Phe NPs was enhanced. In addition, the appearance, clarity of solution, particle size, zeta potential, particle matter, osmolarity, and pH of the product were evaluated to ensure that the NPs were of the required quality. Our approach should prove useful for product and process characterization and quality control in the manufacture of NPs. γ-PGA-Phe NPs are known to be a powerful vaccine adjuvant, so they are expected to undergo clinical development into a practical drug-delivery system. The analytical methods established in this paper should facilitate the reliable and practical quality testing of NP products, thus aiding the clinical development of γ-PGA-Phe-based drug-delivery systems. Moreover, since these analytical methods employ commonly used reagents and chromatographic systems, the methods are expected to be applicable to other NP-based drug-delivery products too.

NPs were completely dissociated into separate γ-PGA-Phe polymeric molecules, which yielded a similar chromatogram to that seen for the NPs





Similar content being viewed by others
References
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–69.
Du C, Zhao J, Fei J, Cui Y, Li J. Assembled microcapsules by doxorubicin and polysaccharide as high effective anticancer drug carriers. Adv Healthcare Mater. 2013;2:1246–51.
Gao L, Cui Y, He Q, Yang Y, Fei J, Li J. Selective recognition of co-assembled thrombin aptamer and docetaxel on mesoporous silica nanoparticles against tumor cell proliferation. Chem Eur J. 2011;17:13170–4.
Yan X, Zhu P, Fei J, Li J. Self-assembly of peptide-inorganic hybrid spheres for adaptive encapsulation of guests. Adv Mater. 2010;22:1283–7.
Li D, Li C, Wang A, He Q, Li J. Hierarchical gold/copolymer nanostructures as hydrophobic nanotanks for drug encapsulation. J Mater Chem. 2010;20:7782–7.
Thomas C, Rawat A, Weeks LH, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8:405–15.
Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori-loaded poly(D,L-lactide-co-glycolide) nanoparticles. Helicobacter. 1999;4:33–9.
Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–64.
Lü JM, Wang X, Muller CM, Wang H, Lin PH, Yao Q. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;4:325–41.
McCall RL, Sirianni RW. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J Vis Exp. 2013;(82):51015. https://doi.org/10.3791/51015.
Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol Pharm. 2013;10:2093–110.
Sokolova V, Knuschke T, Buer J, Westendorf AM, Epple M. Quantitative determination of the composition of multi-shell calcium phosphate-oligonucleotide nanoparticles and their application for the activation of dendritic cells. Acta Biomater. 2011;7:4029–36.
Akagi T, Baba M, Akashi M, Kunugi S. Polymers. In: Yamaoka T, editor. Nanomedicine. Berlin: Springer-Verlag; 2012. p. 31–64.
Akagi T, Kaneko T, Kida T, Akashi M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: potential applications. J Control Release. 2005;108:226–36.
Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, et al. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immun. 2007;178:2979–86.
Uto T, Akagi T, Hamasaki T, Akash M, Baba M. Uptake of biodegradable poly(γ-glutamic acid) nanoparticles and antigen presentation by dendritic cells in vivo. Immunol Lett. 2009;125:46–52.
Himeno A, Akagi T, Uto T, Wang X, Baba M, Ibuki K. Evaluation of the immune response and protective effects of vaccination in rhesus macaques with biodegradable nanoparticles carrying gp120 of human immunodeficiency virus. Vaccine. 2010;28:5377–85.
Okamoto S, Matsuura M, Akagi T, Akashi M, Tanimoto IT, Takahashi T, et al. Poly(γ-glutamic acid) nano-particles combined with mucosal influenza virus hemagglutinin vaccine protects against influenza virus infection in mice. Vaccine. 2009;27:5896–905.
Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007;28:3427–36.
Yamaguchi S, Tatsumi T, Takehara T, Sasakawa A, Yamamoto M, Kohga K, et al. EphA2-derived peptide vaccine with amphiphilic poly(γ-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol Immunother. 2010;59:759–67.
Sagadevan S, Podder J. Investigation of structural, SEM, TEM and dielectric properties of BaTiO3 nanoparticles. J Nano-Electron Phys. 2015;7:04008–1–-4.
Lin WC, Yang MC. Novel silver/poly(vinyl alcohol) nanocomposites for surface-enhanced Raman scattering-active substrates. Macromol Rapid Commun. 2005;26:1942–7.
Pyrz WD, Buttrey DJ. Particle size determination using TEM: a discussion of image acquisition and analysis for the novice microscopist. Langmuir. 2008;24:11350–60.
Ebenstein Y, Nahum E, Banin U. Tapping mode atomic force microscopy for nanoparticle sizing: tip−sample interaction effects. Nano Lett. 2002;2:945–50.
Zhang B, Yan B. Analytical strategies for characterizing nanoparticle’s surface chemistry. Anal Bioanal Chem. 2010;396:973.
Watson GD. Pharmaceutical analysis. 3rd ed. London: Churchill Livingstone; 2012.
Beckett AH, Stenlake JB. Practical pharmaceutical chemistry. 4th ed. New Delhi: CBS Publishers and Distributors Pvt. Ltd.; 1997.
Higuchi T, Hansen B. Pharmaceutical analysis. 3rd ed. New Delhi: CBS Publishers and Distributors; 1997.
Ikeda M, Akagi T, Yasuoka T, Nagao M, Akashi M. Characterization and analytical development for amphiphilic poly(γ-glutamic acid) as raw material of nanoparticle adjuvants. J Pharma Biomed Anal. 2018;150:460–8.
Schwartz M, Krull IS. Analytical regulatory and validation compliance. New York, USA: Marcel Dekker; 1997.
Riley CM, Rosanske TW, editors. Development and validation of analytical methods. Oxford, UK: Pergamon; 1996.
Miller JM, Crowther JB, editors. Analytical chemistry in a GMP environment. New York, USA: Wiley; 2000.
Ermer J, Miller JH, editors. Method validation in pharmaceutical analysis. Weinheim, Germany: Wiley-VCH Verlag; 2005.
Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol Pharm. 2013;10:2093–110.
Kazakevich Y, Loburutto BR. Stationary phases. In: Kazakevich Y, Loburutto BR, editors. HPLC for pharmaceutical scientists. Hoboken, NJ, USA: Wiley and Sons, Inc.; 2007. p. 92–8.
Matsusaki M, Hiwatari K, Higashi M, Kaneko T, Akashi M. Nanosphere induced gene expression in human dendritic cells. Chem Lett. 2004;33:398–9.
Akagi T, Baba M, Akashi M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: potential applications. Polymer. 2007;48:6729–47.
Kaneko T, Higashi M, Matsusaki M, Akagi T, Akashi M. Self-assembled soft nanofibrils of amphipathic polypeptides and their morphological transformation. Chem Mater. 2005;17:2484–6.
Jimenez VL, Leopold MC, Mazzitelli C. HPLC of monolayer-protected gold nanoclusters. Anal Chem. 2003;75:199–206.
Wilcoxon JP, Martin JE, Provencio P. Size distributions of gold nanoclusters studied by liquid chromatography. Langmuir. 2000;16:9912–20.
Kim H, Akagi T, Akashi M. Preparation of size tunable amphiphilic poly(amino acid) nanoparticles. Macromol Biosci. 2009;9:842–8.
Yan H, Hou YF, Niu PF, Zhang K, Shoji T, Tsuboi Y, et al. Biodegradable PLGA nanoparticles loaded with hydrophobic drugs: confocal Raman microspectroscopic characterization. J Mater Chem B. 2015;3:3677–80.
Kim H, Uto T, Akagi T, Baba M, Akashi M. Amphiphilic poly(amino acid) nanoparticles induce size-dependent dendritic cell maturation. Adv Funct Mater. 2010;20:3925–31.
Akagi T, Higashi M, Kaneko T, Kida T, Akashi M. In vitro enzymatic degradation of nanoparticles prepared from hydrophobically-modified poly(gamma-glutamic acid). Macromol Biosci. 2005;5:598–602.
Ghanbarzadeh S, Valizadeh H, Milani PZ. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv Pharm Bull. 2013;3:25–9.
Acknowledgements
We would like to thank Dr. Tetsuo Miwa, Dr. Masami Kusaka, Mr. Tatsuya Yasuoka, Mr. Jun Hirose, Mr. Hikaru Taira (Takeda Pharmaceutical Company, Ltd.), Prof. Masanori Baba (Kagoshima University), and Dr. Tomofumi Uto (University of Miyazaki) for their many suggestions and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ikeda, M., Akagi, T., Nagao, M. et al. Development of analytical methods for evaluating the quality of dissociated and associated amphiphilic poly(γ-glutamic acid) nanoparticles. Anal Bioanal Chem 410, 4445–4457 (2018). https://doi.org/10.1007/s00216-018-1099-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1099-2