Abstract
Endometrial cancer is one of the most commonly diagnosed cancers in women. The search for factors that contribute to the development of cancer cells in reproductive organs should involve the detection of xenoestrogens, in particular zearalenone (ZEA) and its metabolites. Xenoestrogens are endocrine disruptors–ZEA and its metabolites are structurally similar to estrogens (macrocyclic lactone ring) and show high affinity for estrogen receptors. This study proposes a new method for the preparation of samples of human tissues with endometrial cancer by the use of the QuEChERS technique. Analytical parameters such as centrifugation temperature, extraction solvent, and adsorbents were modified to obtain satisfactory recovery for ZEA (R = 82.6%, RSD = 2.9%) and one of its metabolites, α-zearalenol (R = 50.1%, RSD = 3.2%). High-performance liquid chromatography (HPLC) with fluorescence detection and tandem mass spectrometry (LC-QTOF-MS) were used for the identification and quantitative determination of the analyzed compounds. The developed procedure was applied for analyses of human tissues with endometrial cancer. The presence of α-zearalenol was detected in 47 out of the 61 examined tissue samples.

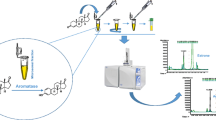
Methodology for isolation and identification of zearalenone and its major metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xenoestrogens are hormonally active substances that are widely distributed in the environment (cigarette smoke, animal feed, water, meat, milk) and exert a negative impact on living organisms. This group of compounds includes zearalenone/fusarium-2 (ZEA/F-2) which occurs mainly in corn cobs, rice, cereals, and wheat [1,2,3,4]. Zearalenone is absorbed from contaminated food shortly after ingestion, and it is rapidly metabolized in the gastrointestinal tract to produce more toxic compounds: α-zearalanol (α-ZAL), α-zearalenol (α-ZEL), β-zearalanol (β-ZAL), and β-zearalenol (β-ZEL). The studies of the biotransformation for ZEA in animals [5,6,7,8] have reported that ZEA is converted to its metabolites, α-ZEL and β-ZEL, which are further converted to α-ZAL and β-ZAL, respectively (Fig. 1). These compounds are endocrine disruptors because ZEA and its metabolites are structurally similar to estrogens (macrocyclic lactone ring) and show high affinity for estrogen receptors (ERα and ERβ) [8]. On the other hand, insignificant quantities of zearalanone are a minor component of the zearalenone complex produced by several species of Fusarium [8].
The adverse effects of ZEA and its metabolites on reproductive organs have been documented in numerous animal studies. In a study of female rats, Turcotte [9] reported an increase in uterine weight in animals administered feed containing ZEA. In the work of Brydl et al. [10], the uterine weight of pigs receiving feed contaminated with ZEA was higher in comparison with the control group, and endometrial hyperplasia was observed in the experimental animals. The results of research [11,12,13,14,15] investigating the effects on ZEA and its metabolites on the reproductive organs of animals constitute the basis for human studies. The presence of ZEA and its metabolites was analyzed by Twarużek et al. [16] in prostate tissues and by Gadzała-Kopciuch et al. [17] in uterine mass tissue (endometrial cancer).
Endometrial cancer in one of the most commonly diagnosed cancers in women. This type of cancer is hormone-dependent, and it is associated with hormonal imbalance. The risk of endometrial cancer is significantly increased by obesity, diabetes, sedentary lifestyle, and infertility [18, 19]. These factors are associated with estrogen stimulation. 17β-estradiol (E2) is a natural estrogen that stimulates the division of uterine cells, whereas progesterone exerts antagonistic effects by inhibiting cell proliferation. In hormonal imbalance, progesterone cannot compensate for the stimulatory effects of estrogen, which could lead to excessive cell division, errors in DNA replication, and carcinogenesis. Xenoestrogens alter estrogen receptors and disrupt estrogen signaling, which leads to cell proliferation. Xenoestrogens accumulate in the uterus and contribute to cancer [18,19,20,21,22].
Caution must be taken when preparing tissue samples for analysis. Tissues are highly heterogeneous; therefore, the sample preparation method must be highly specific and selective for the analyte to be determined. Surgically excised issues have to be secured and frozen. Samples should be prepared directly from frozen tissue. Brand new laboratory glassware should be used to prevent contamination and cross-reactivity [23, 24].
Various tissue preparation methods have been discussed in the literature. One of the proposed methods involves solid-phase extraction with various sorbents, such as octadecyl (C18) [13] or an immunoaffinity column (IAC) [14, 16]. However, these methods are expensive (IAC can be used only once) and time consuming. The cost of isolating the examined compounds with the use of antibodies is high. For this reason, efforts are being made to develop new selective sorbents and effective sample preparation methods that guarantee high recovery at low cost. The QuEChERS technique reduces the number of sample preparation steps and facilitates analyses of numerous samples [25, 26]. The stages of QuEChERS can be modified to detect various compounds, including ZEA and its metabolites, in different matrices. On the other hand, instrumental analysis must show high sensitivity to the tested compounds which are found in biological samples in trace amounts. For this reason, ZEA and its metabolites are increasingly often analyzed by high-performance liquid chromatography coupled with mass spectrometry (LC-MS), which supports analyte identification, while maintaining a low limit of detection and quantification [27]. However, a fluorescence detector is often used due to the specificity of detection and significantly lower matrix effects on ZEA and its metabolites. Undoubtedly, LC-FLD is a less cost-effective solution than LC-MS.
Zearalenone and its metabolites accumulate in reproductive organs, which is why their presence is investigated in human uterine tissues altered by endometrial cancer. This study proposes a new method for the isolation and identification of these compounds with the use of the QuEChERS technique which facilitates the preparation a large number of samples within a short time. High-performance liquid chromatography (HPLC) with fluorescence detection was used for the quantitative determination of zearalenone and its metabolite α-zearalenol extracted from biological samples. Further quantification and confirmatory identification analyses were carried out by ultra-high performance liquid chromatography with tandem mass spectrometry.
Materials and methods
Reagents
The following reagents were used in the study: solvents–methanol, acetonitrile, n-hexane for HPLC, formic acid (LC-MS), ethyl acetate (Avantor Performance Materials Poland S.A., Gliwice), deionized water (Milli-Q Reagent Water (< 10 MΩ cm−1 resistivity) (Merck, Millipore); sorbents–Bondesil PSA, 40 μm (Labstore, Poland), Bakerbond octadecyl (C18, 40 μm, 60 Å), Bakerbond aminopropyl (NH2, 40 μm, 60 Å), Bakerbond 1°,2°-Amino (NH2/NH, 40 μm, 60 Å) from J.T Baker (Avantor Performance Materials Poland); salts–magnesium sulfate anhydrous (99.5% purity), sodium chloride (99.9% purity) (Avantor Performance Materials Poland), sodium citrate monobasic–HOC(COONa)(CH2COOH)2 (99.5% purity), (Sigma-Aldrich, Poland), sodium acetate anhydrous (99.9% purity) (J.T. Baker, Avantor Performance Materials Poland); standards for ZEA (98% purity) and its metabolites–α-ZEL (98% purity), α-ZAL (97% purity), β-ZAL (98% purity), β-ZEL (98% purity); and an internal standard–zearalanone (ZAN: 98% purity) (Sigma-Aldrich, Poland).
The concentrations of standard solutions were 1152 ng/mL ZEA, 960 ng/mL α-ZAL and α-ZEL, and 800 ng/mL β-ZAL and β-ZEL. Solutions with lower concentrations were produced by diluting the working solutions to obtain concentrations of 0.4 to 748 ng/mL in the methanol/water (60:40, v/v) mobile phase.
Equipment
A high-performance liquid chromatography system (Agilent Technologies, model 1100) equipped with a gradient pump, degassing system, thermostatted autosampler, thermostat columns, and DAD and FLD detectors (model 1260) was used in the study. Data were acquired and quantified with the use of OpenLab software.
The stationary phase for chromatographic separation was an ACE 3 C18-AR column (Advanced Chromatography Technologies, 150 × 4.6 mm; dc = 3 μm). The column was kept at a temperature of 30 °C. Chromatographic separation was performed by gradient elution with water (A), acetonitrile (B), and methanol (C) (Table 1). The flow rate was 0.7 mL/min, and injection volume was 40 μL. Analytical wavelength in the fluorescence detector was λex = 270 nm and λem = 440 nm. The fluorescence detector’s photomultiplier tube (PMT) gain was set to 11. Data were collected at a frequency of 4.63 Hz (response time of 2 s).
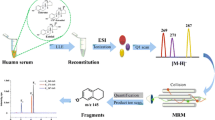
An ultra-high performance liquid chromatography system (model 1260) with a DAD detector, coupled with the quadrupole time-of-flight mass spectrometry (6530 Accurate-Mass QTOF LC/MS; Agilent Technologies), was also used in the study. Data were acquired and quantified with the use of MassHunter Workstation software. Chromatographic separation was performed with a different gradient elution program with water (A) and methanol (B) (Table 1). The elution gradient program for the separation of ZEA and its metabolites in HPLC-FLD differs significantly from that in LC-MS. When the mixtures are separated by LC-MS, compounds are identified based on a selected ion, and chromatographic separation need not be complete. Unfortunately, the resolution in HPLC-FLD should be above 1 in order to be considered complete. The column and the flow rate were identical to those used in HPLC with fluorescence detection. The mass spectrometer was equipped with an ESI source. The mass was tuned and mass calibrated before every analysis day. The following parameters were applied for detection: negative ionization mode (m/z−: α,β-ZAL 321.17; α,β-ZEA 319.15; ZEA 317.14), drying gas temperature–350 °C; drying gas flow rate–10 L/min; nebulizer gas–40 psi; needle voltage–3000 V; fragmentor voltage–100 V.
Tissue specimens were processed in the UW 2070 ultrasonic homogenizer (Bandelin Electronic, Berlin). Samples were evaporated with the MultiVap 8-position Automatic Concentrator (LabTech).
Sample preparation method development
The QuEChERS technique was selected to prepare tissue samples based on the procedure proposed by Woźniak et al. [25]. The method was first tested on animal tissues (fresh poultry muscles from the market). The sample was placed in a 50 mL Falcon tube. Certified tissues with analytically determined content of zearalenone and its metabolites were not available; therefore, samples were prepared with the addition of a reference standard. Samples for analyzing extraction efficiency were spiked with 250 μL of standard solution of ZEA and its metabolites and the internal standard (ZAN), secured with parafilm and refrigerated overnight. On the following day (a), 7 mL of water was added, and the sample was processed in an ultrasonic homogenizer (15 min). In the second variant (b), 10 mL of methanol was added, the sample was homogenized, enriched with methanol extract to 500 μL, and 6.5 mL of water was added to the residue. Ten milliliters of ethyl acetate was added to the Falcon tube (variants a and b), the contents were stirred for 1 min and centrifuged (6000 rpm, 10 min). The upper phase was removed and transferred to a clean 50 mL Falcon tube. Extraction salts were added: 1.5 g of CH3COONa and 6 g of MgSO4. The contents were mixed in a Vortex stirrer (2.5 min), centrifuged (6000 rpm, 10 min), and transferred to a clean Falcon tube. The next stage was dispersive solid-phase extraction (dSPE). The following reagents were added: sorbents–400 mg of PSA and 400 mg of C18, and salts–1200 mg of MgSO4. The contents were mixed in a Vortex stirrer (2.5 min), centrifuged (6000 rpm, 10 min), and transferred to a glass tube. The extract was evaporated under a gentle stream of nitrogen at 60 °C. The residue was dissolved in 3 mL of methanol. The sample was degreased in n-hexane (2 × 5 mL). The methanol phase was evaporated under a stream of nitrogen (60 °C). The residue was reconstructed in 250 μL of the methanol/water (60/40, v/v) mixture.
The dSPE procedure was optimized. The described procedure (a) was used without the tissue model, only for standard solutions of ZEA (532 ng/mL). Eight milliliters of water was transferred to a 50 mL Falcon tube, and 10 mL of ethyl acetate and 250 μL of standard solution of ZEA (532 ng/mL) were added. The effect of different dSPE sorbents and salts (I–400 mg of PSA + 400 mg of C18 + 1200 mg of MgSO4; II–400 mg of C18 + 1200 mg of MgSO4; III–150 mg of C18 + 1200 mg of MgSO4; IV–150 mg of C18 + 1200 mg of sodium citrate monobasic) on the efficiency of recovery was analyzed. The samples were placed in an ultrasonic bath for 15 min. The contents were then centrifuged (6000 rpm, 10 min) to separate the organic–upper layer. The extract was transferred to a glass tube and evaporated under a gentle stream of nitrogen at 60 °C. The residue was dissolved in 250 μL of the methanol/water (60/40, v/v) mixture.
An interesting procedure was proposed by Arroyo-Manzanares et al. [28], and it was tested in this study with several modifications, where dispersive extracts were added to the solid phase (dSPE) and the extracts obtained by QuEChERS were centrifuged at low temperature (4 °C). Arroyo-Manzanares et al. [28] relied on the QuEChERS technique to isolate selected mycotoxins, including ZEA. However, the dispersive liquid-liquid microextraction (DLLME) procedure was necessary due to low concentrations of the isolated aflatoxins (B1, B2, G1, and G2). The cited authors did not investigate metabolites, which is why the above modifications had to be introduced.
A tissue sample was spiked with 250 μL of the standard solution of ZEA (532 ng/mL) and refrigerated overnight. On the following day, 8 mL of water was added to the sample, and the contents were homogenized. Ten milliliters of 5% HCOOH in ACN was added, and the mixture was shaken for 2 min. Salts and sorbents were added in different combinations. In variants A–E, both sorbents and salts were added. In variants F–H, salt was added first, the mixture was shaken and centrifuged to separate the organic layer, the extract was transferred to a clean Falcon tube, and sorbent was added (Table 2). The sample was centrifuged at low temperature (6000 rpm, 10 min, 4 °C). The extract was evaporated under a gentle stream of nitrogen at 60 °C. The residue was dissolved in 250 μL of the methanol/water (60/40, v/v) mixture.
The final version of the developed procedure is shown in Fig. 2, and it was used to prepare samples of human uterine tissue.
Isolation of xenoestrogens from human tissues
Tissue samples weighing 0.5–14 g were obtained from patients with confirmed endometrial cancer and endometrial hyperplasia. If the sample weight was greater than for the spiked samples, the amount of reagents was increased proportionally. Samples were acquired during planned surgical procedures. The collected tissue samples were subjected to histopathological analysis. All experiments were performed in compliance with the relevant laws and institutional guidelines. All research participants expressed their explicit consent to participate in a research study. The study protocol was approved by the Bioethics Committee of the Medical University of Warsaw.
Tissue samples were weighed and transferred to a 50 mL Falcon tube. Eight milliliters of ultrapure water was added, and samples were processed by ultrasonic homogenization. Subsequently, 10 mL of 5% HCOOH in acetonitrile was added, and the contents were stirred for 2 min. The following compounds were added: salts–4 g of MgSO4, 1 g of NaCl, and 1 g of sodium citrate monobasic; and sorbents–400 mg of NH2, 100 mg of C18, and 50 mg of PSA. The contents were shaken for 1 min and processed in an ultrasonic homogenizer for 15 min. The samples were centrifuged at 6000 rpm for 10 min at 4 °C to separate the organic phase. The acetonitrile extract (upper layer) was transferred to a glass tube and evaporated under a gentle stream of nitrogen at 60 °C. The residues were dissolved in 250 μL of MeOH/H2O (60:40, v/v) and passed through a 0.20 μm PTFE syringe membrane filter. The samples were ready for HPLC-FLD and UHPLC-MS/QTOF analyses.
Statistical analysis
The presence of significant differences in the concentrations of ZEA and α-ZEL between cancerous and hyperplastic tissue samples and between age groups was determined by the Student’s t test (p = 0.05). Differences in the frequency of ZEA and α-ZEL detections between healthy and cancerous tissues were determined by the Mann–Whitney U test.
Quality control of samples
Control samples were included in each series: a vial containing the mobile phase and a sample of the internal standard zearalanone (ZAN) which was added to every tissue sample (0.2 ng/g). Standard solutions of the investigated compounds were included in each run in concentrations read from the calibration curve (low, middle, and high points).
Results and discussion
Development of the sample preparation method with the QuEChERS technique
The tested procedure was modeled on the literature [25], and it was characterized by low recovery (R = 22.8%) and poor reproducibility (RSD = 11.3%). Water was replaced with methanol as the solvent for homogenization, which considerably improved reproducibility (RSD = 2.5%), but not recovery (R = 27.7%). Our findings did not approximate the results reported by Woźniak et al. [25] (R = 88.7–119.9%; CV = 9.9–23.6) who had developed the tissue preparation procedure. Repeatability (CV) fluctuated widely. Sample purification and recovery were very low, especially considering the fact that the proposed procedure had been designed specifically for similar types of biological samples, such as tissues.
The causes of considerable analyte loss were investigated. Based on the literature, we checked analyte adsorption when PSA was used as the sorbent. The sorbent remaining from the previous procedure was re-extracted. A small peak corresponding to the retention time of ZEA was observed in the chromatogram. It suggests that dSPE is a critical stage in the QuEChERS method.
The impact of PSA on C18 was checked with standard solutions. When the original set of sorbents (400 mg of PSA and 400 mg of C18) was used, the analyte was adsorbed in 53%. PSA was removed from the dSPE set, and recovery increased by 30%. Recovery reached 87% when the amount of C18 was reduced to 150 mg. A similar result was noted when MgSO4 was replaced with sodium citrate monobasic (R = 85%). The above results indicate that PSA strongly adsorbs ZEA and its metabolites, whereas the opposite conclusions had been drawn by Woźniak et al. [25]. In the cited study, the real recovery values (without IS correction) for resorcylic acid lactones ranged from 50 to 70%.
In the modified method of Arroyo-Manzanares et al. [28], the best results were obtained for variant B (the abbreviations are described in Table 2) where both salts and sorbents were added (R = 75% ± 1.6) and for variant G (Table 2) where salts and sorbents were added separately (R = 80% ± 3.2) (Fig. 3). The same sorbents were used for both variants: 400 mg of NH2 and 100 mg of C18. With a high number of real samples (n = 61), we made several attempts to develop a method with a small number of steps to eliminate the need for numerous reagents, shorten the time of analysis, and reduce the risk of error. Based on the small difference between variants G and B, a decision was made to use both sorbents and salts in further analyses, which would reduce the number of procedural steps, shorten analytical time, and lower costs. Tissues are highly heterogeneous matrices, and extracts may contain other compounds, such as proteins and lipids. The dSPE step was designed to clean up the sample. Our previous research demonstrated that PSA strongly absorbs ZEA, therefore PSA was added for a selected set of sorbents in a much smaller amount (50 mg) than suggested in the literature [25]. The tested dose of PSA did not exert a significant impact on recovery which decreased by 1% (R = 74%). Repeatability was also satisfactory (RSD = 2.9%).
Different variants of dispersive solid-phase extraction (QuEChERS technique) for isolation of ZEA. Variants A–E: salts (4 g of MgSO4, 1 g of NaCl; 1 g of sodium citrate monobasic) and sorbents were added together; variants F–H: salts and sorbents were added separately (abbreviations as described in Table 2)
Recovery in the final procedure
The developed sample preparation procedure and the tissue model were used to determine the recovery of ZEA and its metabolites. The proposed variant of the HPLC-FLD method was used in instrumental analysis.
Recovery was satisfactory for ZEA (R = 82.6%, RSD = 2.9%) and α-ZEL (R = 50.1%, RSD = 3.2%). For these compounds, procedure is selective, therefore these results were used in quantitative analyses of tissue extracts. The recovery for β-ZAL and α-ZAL did not reach the anticipated level. Surprisingly, β-ZAL was characterized by very high LOQ (232 ng/g). For this reason, a quantitative analysis of β-ZAL was not performed. The LOD for α-ZAL was not as high (3.5 ng/g), but the signal was attenuated by the tested tissue. Therefore, a quantitative analysis of α-ZAL was also abandoned. Recovery for β-ZEL was only 26.9%, and reproducibility was low (RSD > 6%). For this reason, β-ZEL was excluded from quantitative analysis. The recovery tests for selected compounds were checked at three concentrations (Table 3). The results for ZEA i α-ZEL was satisfactory because these compounds were expected in real sample (human endometrial tissue), and it was decided to finish optimizing the QuEChERS method at this stage.
Validation of the analytical method
The results of LC and LC-MS analyses were validated based on the following criteria: linearity, precision, accuracy, limit of detection, and limit of quantification. The calibration curves were determined by measuring seven concentrations of the selected compounds in six replicates. The extracts were analyzed, and the standard solutions were injected at two different concentrations. The HPLC-FLD method was linear in a concentration range of 3.91 to 26.48 ng/g (excluding β-ZAL), and the UHPLC-Q-TOF/MS method was linear in the range of 0.06 to 22.08 ng/g. Linearity was satisfactory in all cases (ZEA and four metabolites), as demonstrated by high coefficients of determination (above 0.999). Precision (standard deviation) and reproducibility (coefficient of variation) were determined by calculating the area under the peak. The analyzed method was characterized by high precision (SD < 0.006) and very high reproducibility (RSD < 1%). The limit of detection was determined by a signal-to-noise ratio of 3:1, and the limit of quantification by a signal-to-noise ratio of 10:1 (Table 4). The detection quantification for β-ZAL was very high (LOQ 232.4 ng/g); therefore, this compound was not analyzed by HPLC-FLD due to differences in the fluorescence intensity of those analytes. ZEA and α-ZEL were characterized by the most intensive fluorescence. β-ZEL is less fluorescent because its hydroxyl group is found in the beta position, which makes β-ZEL molecules more stable. The metabolites of α-ZAL and β-ZAL lack the C=C bond in the lactone ring, and they are less fluorescent than ZEA, α-ZEL, and β-ZEL.
The limit of quantification for all analyzed compounds (standard solutions) determined in UHPLC-QTOF/MS did not exceed 0.24 ng/g, and was lower than in HPLC-FLD. However, an analysis of the extracts of model samples (tissues contaminated with a combination of standards) revealed a decrease in peak intensity during mass spectrometry, which automatically increased the limit of detection. The above can be attributed to the matrix effect where a combination of compounds in an extract decreases the effectiveness of ionization. For this reason, analytes were separated in the methanol-water mobile phase (with and without the addition of formic acid) in gradient elution. This modification did not increase the effectiveness of ionization in the analyzed compounds (around 5% increase in intensity). Therefore, in successive stages of the study, liquid chromatography-mass spectrometry was used to perform qualitative analyses and identify the examined compounds.
Analysis of neoplastic tissues
Surgically excised samples of human tissue with endometrial cancer and endometrial hyperplasia were analyzed for the presence of ZEA and its metabolite α-ZEL. The developed sample preparation method and liquid chromatography with FLD detection were used (Fig. 4a-c). The procedure for UHPLC-Q-TOF/MS was applied to confirm the identity of labeled compounds (Fig. 5a–c).
A total of 61 tissue specimens obtained from patients aged 45 to 88 years were analyzed, including 49 specimens with endometrial cancer and 12 specimens with endometrial hyperplasia (Table 5). ZEA and its metabolite α-ZEL were not quantified by HPLC-FLD in any of the samples with endometrial hyperplasia. As shown in Table 5 and Fig. 6, there were no significant differences in α-ZEL concentrations (Student’s t test) between cancerous and hyperplastic tissues or between age groups. The concentration of ZEA reached 44.7 ng/g in HPLC-FLD confirmed by UHPLC-MS/QTOF analysis of one tissue sample with endometrioid adenocarcinoma G1. α-ZEL was detected in 47 tissue samples, and ZEA was identified in 30 samples analyzed by UHPLC-MS/QTOF. The frequency of detection (%) of both analytes differed significantly (Mann–Whitney U test, p < 0.01) between tissues with endometrioid adenocarcinoma and endometrial hyperplasia (Fig. 7).
Conclusions
Human tissues pose a considerable challenge for analysts. The heterogeneity and the unique content of blood and fat in each sample make purification difficult. The QuEChERS technique supports the isolation of the analyzed compound and extract purification with the use of selected sorbents. The results of this study indicate that dSPE is a critical step in the QuEChERS method. Lipids can be effectively removed from a sample with the involvement of selected sorbents, including PSA. PSA is highly useful for preparing tissue extracts. However, PSA should be applied in small amounts to prevent excessive absorption of ZEA and, consequently, low recovery. The most satisfactory results are achieved when the PSA dose is reduced from 400 to 50 mg, and when the amine sorbent (NH2) is applied in the amount of 400 mg. The final analysis of human tissue samples supported efficient recovery of the two tested compounds, ZEA and α-ZEL. A total of 61 tissue specimens (body of the uterus) were analyzed, including 49 specimens with endometrial cancer and 12 specimens with endometrial hyperplasia. None of the examined compounds were found in quantitative analyses of tissues obtained from healthy women. Mass spectrometry revealed the presence of α-ZEL in four samples and ZEA in ten samples. Endometrial cancer and the presence of α-ZEL were detected in more than 55% of the examined tissues. Our findings show that ZEA and its metabolites tend to accumulate in reproductive organs. Higher concentrations of the analyzed compounds in tissues with tumor lesions suggest that ZEA and its metabolites could significantly contribute to the proliferation of tumor cells in the uterus.
References
Dzuman Z, Zachariasova M, Lacina O, Veprikova Z, Slavikova P, Hajslova J. A rugged high-throughput analytical approach for the determination and quantification of multiple mycotoxins in complex food matrices. Talanta. 2014;121:263–72.
Wu J, Zhao R, Chen B, Yang M. Determination of zearalenone in barley by high-performance liquid chromatography coupled with evaporative light scattering detection and natural occurrence of zearalenone in functional food. Food Chem. 2011;126:1508–11.
Fucic A, Gamulin M, Ferencic Z, Katic J, Krayer von Krauss M, Bartonova A, et al. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas and brain. Environ Health. 2012;11:1–2.
Hird SJ, Lau BP-Y, Schuhmacher R, Krska R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. TrAC. 2014;59:59–72.
Zinedine A, Soriano JM, Moltó JC, Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone. An oestrogenic mycotoxin. Food & Chem Toxicol. 2007;45:2–7.
Binder SB, Schwartz-Zimmermann HE, Varga E, Bichl G, Michlmayr H, Adam G, Berthiller F. Metabolism of zearalenone and its major modified forms in pigs. Toxins (Basel). 2017; 8;9(2).
Mukherjee D, Royce SG, Alexander JA, Buckley B, Isukapalli SS, Bandera EV, et al. Physiologically-based toxicokinetic modeling of zearalenone and its metabolites: application to the jersey girl study. PLoS One. 2014;9(12):e113632.
Mizutani K, Nagatomi Y, Mochizuki N. Metabolism of zearalenone in the course of beer fermentation. Toxins. 2011;3(2):134–41.
Turcotte JC, Hunt JB, Blaustein JD. Estrogenic effects of zearalenone on the expression of progestin receptors and sexual behavior in female rats. Horm Behav. 2005;47:178–84.
Brydl E, Vanyi A, Glavits R, Konyves L, Rafai P. Reduction of the oestrogenic effect of zearalenone in pigs by a feed additive. Acta Vet Brno. 2014;83:33–8.
Stopa E, Gajecka M, Babinska I, Zielonka L, Gajecki M. The effect of experimental exposure to low doses of zearalenone on uterine histology and morphometry in prepubertal bitches. Theriogenology. 2014;82:537–45.
Chen XX, Yang CW, Huang LB, Niu QS, Jiang SZ, Chi F. Zearalenone altered the serum hormones, morphologic and apoptotic measurements of genital organs in post-weaning gilts. Asian-Australas J Anim Sci. 2015;28(2):171–9.
Jodlbauer J, Zollner P, Linder W. Determination of zearalenone and its metabolites in urine and tissue samples of cow and pig by LC-MS/MS. Micotoxin Res. 2000;16(2):174–8.
Woźny M, Oberski K, Jakimiuk E, Gustian M, Brzuzan P. Zearalenone contamination in rainbow trout farms in north-eastern Poland. Aquaculture. 2013;416-417:209–11.
Gajęcka M, Sławuta P, Nicpoń J, Kołacz R, Kiełbowicz Z, Zielona Ł, et al. Zearalenone and its metabolites in the tissues of female wild boars exposed per os to mycotoxins. Toxicon. 2016;114:1–12.
Twarużek M. Zearalenone and its metabolites in plasma, urine and tissues of the prostate in men with normal, hyperplastic and malignant modified (in Polish). Bydgoszcz: Kazimierz Wielki University Publishing House; 2013.
Gadzała-Kopciuch R, Cendrowski K, Cesarz A, Kiełbasa P, Buszewski B. Determination of zearalenone and its metabolites in endometrial cancer by coupled separation techniques. Anal Bioanal Chem. 2011;401:2069–70.
Biela A, Pacholska-Bogalska J. Hormone-dependent carcinomas in women (in Polish). Nowa Medycyna. 2012;4:76–82.
Boyle P, Levin B. Endometrial Cancer. Lyon: Word Cancer Report, IARC Press; 2008. pp.428–30.
Kreizman-Shefer H, Pricop J, Goldman S, Elmalah I, Shalev E. Distribution of estrogen and progesterone receptors isoforms in endometrial cancer. Diagnostic Pathol. 2014;9:1–2.
Bonneau C, Perrin M, Koskas M, Genin AS, Rouzier R. Epidemiology and risk factors for cancer of the uterus (article in French). Rev Prat. 2014;64:774–9.
Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–8.
De Petris L, Pernemalm M, Elmberger G, Bergman P, Orre L, Lewensohn R, et al. Novel method for sample preparation of fresh lung cancer tissue for proteomics by tumor cell enrichment and removal of blood contaminants. Proteome Sci. 2010;8(9):1–2.
Yo C, Cohen LH. Tissue sample preparation—not the same old grind. LC-GC Europe. 2004;17(2):2–6.
Wozniak B, Matraszek-Zuchowska I, Zmudzki J. Determination of stilbenes and resorcylic acid lactones in bovine, porcine and poultry tissue by liquid chromatography-negative ion electrospray mass spectrometry and QuEChERS for sample preparation. J Chromatogr B. 2013;940:15–23.
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–31.
Sforza S, Dall’Asta C, Marchelli R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom Rev. 2006;25(1):67–8.
Arroyo-Manzanares N, Huerras-Perez J, Gamiz-Gracia L, Cracia-Campana AMA. New approach in sample treatment combined with UHPLC–MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta. 2013;115(1):61–7.
Acknowledgments
The work was financially supported by the National Science Centre as part of the OPUS project No. 153119 (2011/01/B/ST4/00543) and the MAESTRO project No. 2014/14/A/ST4/00641.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in compliance with the relevant laws and institutional guidelines. All research participants expressed their explicit consent to participate in a research study. The study protocol was approved by the Bioethics Committee of the Medical University of Warsaw.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pajewska, M., Łojko, M., Cendrowski, K. et al. The determination of zearalenone and its major metabolites in endometrial cancer tissues. Anal Bioanal Chem 410, 1571–1582 (2018). https://doi.org/10.1007/s00216-017-0807-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0807-7