Abstract
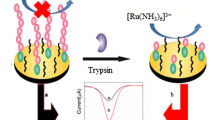
Peptide self-assembly holds tremendous promise for a range of applications in chemistry and biology. In the work reported here, we explored the potential functions of peptide self-assembly in electrochemical bioanalysis by developing a peptide self-assembly assisted signal labeling strategy for assaying protease activity. The fundamental principle of this assay is that target-protease-catalyzed specific proteolytic cleavage blocks self-assembly between the probe peptide and signal peptide, thus preventing the signal labeling of electroactive silver nanoparticles on the electrode surface, which in turn causes the electrochemical signal to decrease. Using trypsin as an example protease target, the linear range of this assay was found to be 1 ng mL−1 to 100 mg mL−1, and its detection limit was 0.032 ng mL−1, which are better than the corresponding parameters for previously reported assays. Further experiments also highlighted the good selectivity of the assay method and demonstrated its usability when applied to serum samples. Therefore, this report not only introduces a valuable tool for assaying protease activity, but it also promotes the utilization of peptide self-assembly in electrochemical bioanalysis, as this approach has great potential for practical use in the future.





Similar content being viewed by others
References
Labib M, Sargent EH, Kelley SO. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem Rev. 2016;116:9001–90.
Ma C, Liu HY, Zhang LN, Li H, Yan M, Song RX, et al. Multiplexed aptasensor for simultaneous detection of carcinoembryonic antigen and mucin-1 based on metal ion electrochemical labels and Ru(NH3)6 3+ electronic wires. Biosens Bioelectron. 2018;99:8–13.
Xu YY, Liu L, Wang ZY, Dai ZH. Stable and reusable electrochemical biosensor for poly(ADP-ribose) polymerase and its inhibitor based on enzyme-initiated auto-PARylation. ACS Appl Mater Inter. 2016;8:18669–74.
Xie S, Yuan Y, Chai Y, Yuan R. Tracing phosphate ions generated during loop-mediated isothermal amplification for electrochemical detection of Nosema bombycis genomic DNA PTP1. Anal Chem. 2015;87:10268–74.
Carter CJ, Ackerson CJ, Feldheim DL. Unusual reactivity of a silver mineralizing peptide. ACS Nano. 2010;4:3883–8.
Merrill NA, Nitka TT, McKee EM, Merino KC, Drummy LF, Lee S, et al. Effects of metal composition and ratio on peptide-templated multimetallic PdPt nanomaterials. ACS Appl Mater Inter. 2017;9:8030–40.
Bhandari R, Knecht MR. Synthesis, characterization, and catalytic application of networked au nanostructures fabricated using peptide templates. Catal Sci Technol. 2012;2:1360–6.
Wang J, Zhao X, Li J, Kuang X, Fan Y, Wei G, et al. Electrostatic assembly of peptide nanofiber–biomimetic silver nanowires onto graphene for electrochemical sensors. ACS Macro Lett. 2014;3:529–33.
Yang Y, Liang Y, Zhang C. Label-free and homogenous detection of caspase-3-like proteases by disrupting homodimerization-directed bipartite tetracysteine display. Anal Chem. 2017;89:4055–61.
Hata R, Nonaka H, Takakusagi Y, Ichikawa K, Sando S. Design of a hyperpolarized molecular probe for detection of aminopeptidase N activity. Angew Chem Int Ed. 2016;128:1797–800.
Meng FY, Tang CH, Wang BD, Liu T, Zhu XL, Miao P. Peptide and carbon nanotubes assisted detection of apoptosis by square wave voltammetry. Electrochim Acta. 2016;199:142–6.
Mascini M, Palchetti I, Tombelli S. Nucleic acid peptide aptamers: fundamentals and bioanalytical aspects. Angew Chem Int Ed. 2012;51:1316–32.
Liu T, Meng FY, Cheng WB, Sun HX, Luo Y, Tang YG, et al. Preparation of a peptide-modified electrode for capture and voltammetric determination of endotoxin. ACS Omega. 2017;2:2469–73.
Li H, Li WW, Liu FZ, Wang ZX, Li GX, Karamanos Y. Detection of tumor invasive biomarker using a peptamer of signal conversion and signal amplification. Anal Chem. 2016;88:3662–8.
Parnsubsakul A, Safitri RE, Rijiravanich P, Surareungchai W. Electrochemical assay of proteolytically active prostate specific antigen based on anodic stripping voltammetry of silver enhanced gold nanoparticle labels. J Electroanal Chem. 2017;785:125–30.
Qu F, Yang M, Rasooly A. Dual signal amplification electrochemical biosensor for monitoring the activity and inhibition of the Alzheimer’s related protease β-secretase. Anal Chem. 2016;88:10559–65.
De Sandis E, Ryadnov MG. Peptide self-assembly for nanomaterials: the old new kid on the block. Chem Soc Rev. 2015;44:8288–300.
Burgess NC, Sharp TH, Thomas F, Wood CW, Thomson AR, Zaccai NR, et al. Modular design of self-assembling peptide-based nanotubes. J Am Chem Soc. 2015;137:10554–62.
Mandal D, Shirazi AN, Parang K. Self-assembly of peptides to nanostructures. Org Biomol Chem. 2014;12:3544–61.
Wang JH, Qin YQ, Li JC, Zhou X, Yao Y, Wang CL, et al. Förster resonance energy transfer analysis of quantum dots and peptide self-assembly inside a capillary. Sensor Actuat B. 2016;227:619–25.
Moitra P, Kumar K, Kondaiah P, Bhattacharya S. Efficacious anticancer drug delivery mediated by a pH-sensitive self-assembly of a conserved tripeptide derived from tyrosine kinase NGF receptor. Angew Chem Int Ed. 2014;53:1113–7.
Liu K, Xing RR, Zou QL, Ma GH, Mohwald H, Yan XH. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew Chem Int Ed. 2016;55:3036–9.
Li LL, Qi GB, Yu FQ, Liu SJ, Wang H. An adaptive biointerface from self-assembled functional peptides for tissue engineering. Adv Mater. 2015;27:3181–8.
Choi SY, Bertram S, Glowacka I, Park YW, Pohlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol Med. 2009;15:303–12.
Krzeminska A, Moliner V, Świderek K. Dynamic and electrostatic effects on the reaction catalyzed by HIV-1 protease. J Am Chem Soc. 2016;138:16283–98.
Lu S, Kanekura K, Hara T, Mahadevan J, Spears LD, Oslowski CM, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci USA. 2014;111:E5292–301.
Huber R, Bode W. Structural basis of the activation and action of trypsin. Acc Chem Res. 1978;11:114–22.
Kang OH, Jeong HJ, Kim DK, Choi SC, Kim TH, Nah YH, et al. Trypsin induces tumour necrosis factor-alpha secretion from a human leukemic mast cell line. Cell Biochem Funct. 2003;21:161–7.
Huang HL, Hsing HW, Lai TC, Chen YW, Lee TR, Chan HT, et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J Biomed Sci. 2010;17:36.
Zhu X, Feng C, Ye Z, Chen Y, Li G. Fabrication of magneto-controlled moveable architecture to develop reusable electrochemical biosensors. Sci Rep. 2014;4:4169.
Setua S, Menon D, Asok A, Nair S, Koyakutty M. Folate receptor targeted, rare-earth oxide nanocrystals for bi-modal fluorescence and magnetic imaging of cancer cells. Biomaterials. 2010;31:714–29.
Tan YN, Lee JY, Wang DIC. Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc. 2010;132:5677–86.
Ray S, Das AK, Drew MGB, Banerjee A. A short water-soluble self-assembling peptide forms amyloid-like fibrils. Chem Commun 2006;4230–4232.
Cao Y, Chen WW, Han P, Wang ZX, Li GX. Target-driven self-assembly of stacking deoxyribonucleic acids for highly sensitive assay of proteins. Anal Chim Acta. 2015;890:1–6.
Miao P, Liu T, Li XX, Ning LM, Yin J, Han K. Highly sensitive, label-free colorimetric assay of trypsin using silver nanoparticles. Biosens Bioelectron. 2013;49:20–4.
Zhang LF, Du JX. A sensitive and label-free trypsin colorimetric sensor with cytochrome c as a substrate. Biosens Bioelectron. 2016;79:347–52.
Duan C, Alibakhshi MA, Kim DK, Brown CM, Craik CS, Majumdar A. Label-free electrical detection of enzymatic reactions in nanochannels. ACS Nano. 2016;10:7476–84.
Xia T, Ma Q, Hu T, Su X. A novel magnetic/photoluminescence bifunctional nanohybrid for the determination of trypsin. Talanta. 2017;170:286–90.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 81671781, 81401489, 31200745), and the Science and Technology Planning Project of Taicang (grant no. TC2015YYCX02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Miao, X., Yu, H., Gu, Z. et al. Peptide self-assembly assisted signal labeling for an electrochemical assay of protease activity. Anal Bioanal Chem 409, 6723–6730 (2017). https://doi.org/10.1007/s00216-017-0636-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0636-8