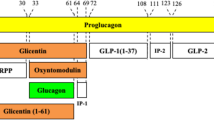
Abstract
Accurate quantification of plasma glucagon levels in humans is necessary for understanding the physiological and pathological importance of glucagon. Although several immunoassays for glucagon are available, they provide inconsistent glucagon values owing to cross-reactivity of the antibodies with peptides other than glucagon. To overcome this limitation, we developed a novel method to measure glucagon levels by a liquid chromatography (LC)-high-resolution mass spectrometry (HRMS) assay via parallel reaction monitoring (PRM) without immunoaffinity enrichment. Using stable isotope-labeled glucagon as an internal standard and 200 μL of plasma, the lower limit of quantification was 0.5 pM. This method was applied to measure plasma glucagon levels during the oral glucose tolerance test (OGTT) and meal tolerance test (MTT) in healthy volunteers, and its results were compared with those of sandwich enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA). During the OGTT, this method showed significant suppression of plasma glucagon levels, and similar patterns were observed with sandwich ELISA and RIA. In contrast, during the MTT, plasma glucagon levels were slightly elevated according to the LC-MS/MS and sandwich ELISA results and were reduced according to RIA results. Our newly developed LC-MS/MS method overcomes a lack of specificity among currently available immunoassays for glucagon and may contribute to a better understanding of the importance of glucagon.

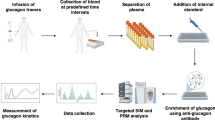
Flowchart for the extraction and quantification of glucagon in human plasma, and plasma glucagon responses in healthy volunteers quantified by the present LC-MS/MS, sandwich ELISA, and RIA during OGTT and MTT



Similar content being viewed by others
Abbreviations
- CV:
-
Coefficient of variation
- ESI:
-
Electrospray ionization
- HRMS:
-
High-resolution mass spectrometry
- IS:
-
Internal standard
- LLOQ:
-
Lower limit of quantification
- MTT:
-
Meal tolerance test
- OGTT:
-
Oral glucose tolerance test
- PP:
-
Protein precipitation
- PRM:
-
Parallel reaction monitoring
- RE:
-
Relative error
- SPE:
-
Solid-phase extraction
References
Mighiu PI, Yue JTY, Filippi BM, Abraham MA, Chari M, Lam CK, et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med. 2013;19(6):766–72.
Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12.
Unger RH, Eisentraut AM, McCall MS, Keller S. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med. 1959;102:621–3.
Bataille D, Dalle S. The forgotten members of the glucagon family. Diabetes Res Clin Pract. 2014;106(1):1–10.
Bak MJ, Albrechtsen NW, Pedersen J, Hartmann B, Christensen M, Vilsbøll T, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170(4):529–38.
Holst JJ, Christensen M, Lund A, de Heer J, Svendsen B, Kielgast U, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab. 2011;13(Suppl 1):89–94.
Albrechtsen NJW, Hartmann B, Veedfald S, Windeløv JA, Plamboeck A, Bojsen-Møller KN, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919–26.
Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme-linked immunosorbent assay. J Diabetes Invest. 2016;7(3):324–31.
Chambers EE, Lame ME, Rainville PD, Murphy J, Johnson J, Fountain KJ, et al. Practical applications of integrated microfluidics for peptide quantification. Bioanalysis. 2015;7(7):857–67.
Chappell DL, Lee AYH, Castro-Perez J, Zhou H, Roddy TP, Lassman ME, et al. An ultrasensitive method for the quantitation of active and inactive GLP-1 in human plasma via immunoaffinity LC-MS/MS. Bioanalysis. 2014;6(1):33–42.
Lee AYH, Chappell DL, Bak MJ, Judo M, Liang L, Churakova T, et al. Multiplexed quantification of proglucagon-derived peptides by immunoaffinity enrichment and tandem mass spectrometry after a meal tolerance test. Clin Chem. 2016;62(1):227–35.
Sano S, Tagami S, Hashimoto Y, Yoshizawa-Kumagaye K, Tsunemi M, Okochi M, et al. Absolute quantitation of low abundance plasma APL1β peptides at sub-fmol/mL level by SRM/MRM without immunoaffinity enrichment. J Proteome Res. 2014;13(2):1012–20.
Grund B, Marvin L, Rochat B. Quantitative performance of a quadrupole-orbitrap-MS in targeted LC-MS determinations of small molecules. J Pharm Biomed Anal. 2016;124:48–56.
Miyachi A, Murase T, Yamada Y, Osonoi T, Harada K. Quantitative analytical method for determining the levels of gastric inhibitory polypeptides GIP1–42 and GIP3–42 in human plasma using LC-MS/MS/MS. J Proteome Res. 2013;12(6):2690–9.
Auld CA. White paper “Glucagon measurement—addressing long-standing analytical challenges.” 2015. https://www.mercodia.se. Accessed 24 Oct 2016.
Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol. 2008;4(222):1–14.
Bagger JI, Knop FK, Lund A, Holst JJ, Vilsbøll T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia. 2014;57(8):1720–5.
Acknowledgements
We are grateful to the members of Thermo Fisher Scientific and to T. Murase, S. Yamashita, and H. Hashimoto for their fruitful discussions and technical support. We also thank the members of the Kitamura Laboratory for the discussion of the data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All participants gave oral and written consent. The study was approved by the Ethics Committee of Gunma University and was conducted in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 261 kb)
Rights and permissions
About this article
Cite this article
Miyachi, A., Kobayashi, M., Mieno, E. et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 409, 5911–5918 (2017). https://doi.org/10.1007/s00216-017-0534-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0534-0