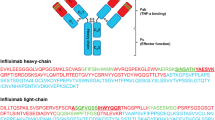
Abstract
Monoclonal antibody-based therapeutic agents (antibody drugs) have attracted considerable attention as a new type of drug. Concomitantly, the use of quantitative approaches for characterizing antibody drugs, such as liquid chromatography (LC)-mass spectrometry (MS), has increased. Generally, selective quantification of antibody drugs is done using unique peptides from variable regions (V H and V L) as surrogate peptides. Further, numerous internal standards (ISs) such as stable isotope-labeled (SIL)-intact proteins and SIL-surrogate peptides are used. However, developing LC-MS methodology for characterizing antibody drugs is time-consuming and costly. Therefore, LC-MS is difficult to apply for this purpose, particularly during the drug discovery stage when numerous candidates must be evaluated. Here, we demonstrate an efficient approach to developing a quantitative LC/electrospray ionization (ESI)-selected reaction monitoring (SRM)/MS method for characterizing antibody drugs. The approach consists of the following features: (i) standard peptides or SIL-IS are not required; (ii) a peptide from the homologous monoclonal antibody serves as an IS; (iii) method development is monitored using a spiked plasma sample and one quantitative MS analysis; and (iv) three predicted SRM assays are performed to optimize quantitative SRM conditions such as transition, collision energy, and declustering potential values. Using this strategy, we developed quantitative SRM methods for infliximab, alemtuzumab, and bevacizumab with sufficient precision (<20%)/accuracy (<±20%) for use in the drug discovery stage. We have also demonstrated that choosing a higher homologous peptide pair (from analyte mAb/IS mAb) is necessary to obtain the sufficient precision and accuracy.

ᅟ





Similar content being viewed by others
Abbreviations
- CDR:
-
Complementarity-determining region
- CE:
-
Collision energy
- CXP:
-
Collision cell exit potential
- DP:
-
Declustering potential
- D-PBS:
-
Dulbecco’s phosphate-buffered saline
- DTT:
-
Dithiothreitol
- ESI:
-
Electrospray ionization
- EP:
-
Entrance potential
- IAA:
-
Iodoacetamide
- IS:
-
Internal standard
- LBA:
-
Ligand binding assay
- LC:
-
Liquid chromatography
- mAb:
-
Monoclonal antibody
- MS:
-
Mass spectrometry
- pSRM:
-
Predicted SRM
- QC:
-
Quality control
- SRM:
-
Selected reaction monitoring
- SIL:
-
Stable isotope-labeled
- V H :
-
Heavy-chain variable region
- V L :
-
Light-chain variable region
References
Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12:615–22. doi:10.1016/j.coph.2012.08.001.
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad SciUSA. 1996;93:5512–6. (http://www.pnas.org/content/93/11/5512.full.pdf)
Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–6. doi:10.1002/eji.1830260327.
Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–8. doi:10.1046/j.1365-2567.1996.d01-775.x.
Philippidis A. The top 25 best-selling drugs of 2014, Genetic Engineering & Biotechnology News, Feb 23, 2015. (http://www.genengnews.com/keywordsandtools/print/3/37387/).
DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87:272–7. doi:10.1038/clpt.2009.295.
Li F, Fast DD, Michael S. Absolute quantitation of protein therapeutics in biological matrices by enzymatic digestion and LC-MS. Bioanalysis. 2011;3:2459–80. doi:10.4155/bio.11.237.
van den Broek I, Niessen WM, van Dongen WD. Bioanalytical LC-MS/MS of protein-based biopharmaceuticals. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;929:161–79. doi:10.1016/j.jchromb.2013.04.030.
An B, Zhang M, Qu J. Toward sensitive and accurate analysis of antibody biotherapeutics by liquid chromatography coupled with mass spectrometry. Drug Metab Dispos. 2014;42:1858–66. doi:10.1124/dmd.114.058917.
Hoofnagle AN, Wener MH. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009;347:3–11. doi:10.1016/j.jim.2009.06.003.
Whitehouse CM, Dreyer RN, Yamashita M, Fenn JB. Electrospray interface for liquid chromatographs and mass spectrometers. Anal Chem. 1985;57:675–9. doi:10.1021/ac00280a023.
Li H, Ortiz R, Tran LT, Salimi-Moosavi H, Malella J, James CA, et al. Simultaneous analysis of multiple monoclonal antibody biotherapeutics by LC-MS/MS method in rat plasma following cassette-dosing. AAPS J. 2013;15:337–46. doi:10.1208/s12248-012-9435-5.
Jiang H, Zeng J, Titsch C, Voronin K, Akinsanya B, Luo L, et al. Fully validated LC-MS/MS assay for the simultaneous quantitation of coadministered therapeutic antibodies in cynomolgus monkey serum. Anal Chem. 2013;15:9859–67. doi:10.1021/ac402420v.
Furlong MT, Ouyang Z, Wu S, Tamura J, Olah T, Tymiak A, et al. A universal surrogate peptide to enable LC-MS/MS bioanalysis of a diversity of human monoclonal antibody and human Fc-fusion protein drug candidates in pre-clinical animal studies. Biomed Chromatogr. 2012;26:1024–32. doi:10.1002/bmc.2759.
Furlong MT, Titsch C, Xu W, Jiang H, Jemal M, Zeng J. An exploratory universal LC-MS/MS assay for bioanalysis of hinge region-stabilized human IgG4 mAbs in clinical studies. Bioanalysis. 2014;6:1747–58. doi:10.4155/bio.14.64.
Furlong MT, Zhao S, Mylott W, Jenkins R, Gao M, Hegde V, et al. Dual universal peptide approach to bioanalysis of human monoclonal antibody protein drug candidates in animal studies. Bioanalysis. 2013;5:1363–76. doi:10.4155/bio.13.55.
Zhang Q, Spellman DS, Song Y, Choi B, Hatcher NG, Tomazela D, et al. Generic automated method for liquid chromatography-multiple reaction monitoring mass spectrometry based monoclonal antibody quantitation for preclinical pharmacokinetic studies. Anal Chem. 2014;86:8776–84. doi:10.1021/ac5019827.
Dubois M, Fenaille F, Clement G, Lechmann M, Tabet JC, Ezan E, et al. Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal Chem. 2008;80:1737–445. doi:10.1021/ac7021234.
Fernández Ocaña M, James IT, Kabir M, Grace C, Yuan G, Martin SW, et al. Clinical pharmacokinetic assessment of an anti-MAdCAM monoclonal antibody therapeutic by LC-MS/MS. Anal Chem. 2012;84:5959–67. doi:10.1021/ac300600f.
Becker JO, Hoofnagle AN. Replacing immunoassays with tryptic digestion-peptide immunoaffinity enrichment and LC-MS/MS. Bioanalysis. 2012;4:281–90. doi:10.4155/bio.11.319.
Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, et al. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25:1469–83. doi:10.1007/s11095-008-9532-4.
Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F, et al. Anal Chem. 2008;80:1290–6. doi:10.1021/ac702115b.
Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K, Lehmann N, et al. Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem. 2008;80:4200–7. doi:10.1021/ac800205s.
Li H, Ortiz R, Tran L, Hall M, Spahr C, Walker K, et al. General LC-MS/MS method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal Chem. 2012;84:1267–73. doi:10.1021/ac202792n.
Nouri-Nigjeh E, Zhang M, Ji T, Yu H, An B, Duan X, et al. Effects of calibration approaches on the accuracy for LC-MS targeted quantification of therapeutic protein. Anal Chem. 2014;86:3575–84. doi:10.1021/ac5001477.
Liu G, Ji QC, Dodge R, Sun H, Shuster D, Zhao Q, et al. Liquid chromatography coupled with tandem mass spectrometry for the bioanalysis of proteins in drug development: practical considerations in assay development and validation. J Chromatogr A. 2013;1284:155–62. doi:10.1016/j.chroma.2013.02.016.
Yang Z, Hayes M, Fang X, Daley MP, Ettenberg S, Tse FL. LC-MS/MS approach for quantification of therapeutic proteins in plasma using a protein internal standard and 2D-solid-phase extraction cleanup. Anal Chem. 2007;79:9294–301. doi:10.1021/ac0712502.
Lame M, Yang H, Naughton S, Chambers E. An intact murine monoclonal antibody for use as a generic internal standard and workflow check standard in protein bioanalysis studies. Waters Co., Milford, MA, USA, Waters application note 720005543 ( http://www.waters.com/webassets/cms/library/docs/720005543en.pdf).
Jenkins R, Duggan JX, Aubry AF, Zeng J, Lee JW, Cojocaru L, et al. Recommendations for validation of LC-MS/MS bioanalytical methods for protein biotherapeutics. AAPS J. 2015;17:1–16. doi:10.1124/dmd.114.058917.
Lu Q, Zheng X, McIntosh T, Davis H, Nemeth JF, Pendley C, et al. Development of different analysis platforms with LC-MS for pharmacokinetic studies of protein drugs. Anal Chem. 2009;81:8715–23. doi:10.1021/ac901991x.
Yuan L, Arnold ME, Aubry AF, Ji QC. Simple and efficient digestion of a monoclonal antibody in serum using pellet digestion: comparison with traditional digestion methods in LC-MS/MS bioanalysis. Bioanalysis. 2012;4:2887–96. doi:10.4155/bio.12.284.
Yuan L, Aubry AF, Arnold ME, Ji QC. Systematic investigation of orthogonal SPE sample preparation for the LC-MS/MS bioanalysis of a monoclonal antibody after pellet digestion. Bioanalysis. 2013;5:2379–91. doi:10.4155/bio.13.224.
Osaki F, Goto T, Lee SH, Oe T. Predicted multiple selected reaction monitoring to screen activated drug-mediated modifications on human serum albumin. Anal Biochem. 2014;449:59–67. doi:10.1016/j.ab.2013.12.016.
McGinley M, Jarrett D, Layne J, Chitty M, Farkas T. Optimising core-shell UHPLC columns for improving protein and peptide separations. Chromatography Today 4 2011;33–36. (http://www.chromatographytoday.com/articles/hplc-uhplc/31/michael_mcginley_deborah_jarrett_jeff_layne_mike_chitty_and_tivadar_farkas/optimising_core-shell_uhplc_columns_for_improving_protein_and_peptide_separations/1099/).
Duan X, Abuqayyas L, Dai L, Balthasar JP, Qu J. High-throughput method development for sensitive, accurate, and reproducible quantification of therapeutic monoclonal antibodies in tissues using orthogonal array optimization and nano liquid chromatography/selected reaction monitoring mass spectrometry. Anal Chem. 2012;84:4373–82. doi:10.1021/ac2034166.
Duan X, Dai L, Chen SC, Balthasar JP, Qu J. Nano-scale liquid chromatography/mass spectrometry and on-the-fly orthogonal array optimization for quantification of therapeutic monoclonal antibodies and the application in preclinical analysis. J Chromatogr A. 2012;1251:63–73. doi:10.1016/j.chroma.2012.06.007.
Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–52. doi:10.1038/nnnnn2747.
Acknowledgements
The authors thank Aiji Miyashita, Masako Furutani, Fujiko Takamura, and Dr. Tetsu Saito of Astellas Pharma Inc. for their valuable scientific discussions and Masamichi Yuda and Masashi Kawasaki of Astellas Pharma Inc. for providing antibodies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 5012 kb)
Rights and permissions
About this article
Cite this article
Osaki, F., Tabata, K. & Oe, T. Quantitative LC/ESI-SRM/MS of antibody biopharmaceuticals: use of a homologous antibody as an internal standard and three-step method development. Anal Bioanal Chem 409, 5523–5532 (2017). https://doi.org/10.1007/s00216-017-0488-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0488-2