Abstract
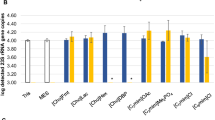
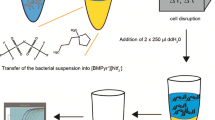
In this study, a series of magnetic ionic liquids (MILs) were investigated for the extraction and preconcentration of bacteria from aqueous samples. By dispersing small volumes (e.g., 15 μL) of MIL within an aqueous cell suspension, bacteria were rapidly extracted and isolated using a magnetic field. Of the seven hydrophobic MILs examined, the trihexyl(tetradecyl)phosphonium Ni(II) hexafluoroacetylacetonate ([P66614 +][Ni(hfacac)3 −]) MIL exhibited the greatest enrichment of viable Escherichia coli K12 when coupled with microbiological culture as the detection method. The MIL-based strategy was applied for the preconcentration of E. coli from aqueous samples to obtain enrichment factors (E F) as high as 44.6 in less than 10 min. The MIL extraction approach was also interfaced with polymerase chain reaction (PCR) amplification where the positive detection of E. coli was achieved with the [P66614 +][Co(hfacac)3 −], [P66614 +][Ni(hfacac)3 −], [P66614 +][Dy(hfacac)4 −], and [P66614 +][Nd(hfacac)4 −] MILs. While direct sampling of an aqueous cell suspension at a concentration of 1.68 × 104 colony-forming units (CFUs) mL−1 yielded no amplicon when subjected to PCR, extraction of the sample with the [P66614 +][Ni(hfacac)3 −] MIL under optimized conditions provided sufficient enrichment of E. coli for amplicon detection. Importantly, the enrichment of bacteria using the Ni(II)-, Co(II)-, and Dy(III)-based MILs was compatible with real-time quantitative PCR amplification to dramatically improve sample throughput and lower detection limits to 1.0 × 102 CFUs mL−1. The MIL-based method is much faster than existing enrichment approaches that typically require 24-h cultivation times prior to detection and could potentially be applied for the preconcentration of a variety of Gram-negative bacteria from aqueous samples.

Magnetic ionic liquid solvents rapidly preconcentrate viable E. coli cells for unambiguous pathogen detection using microbiological culture and qPCR







Similar content being viewed by others
References
Cabral JPS. Water microbiology. Bacterial pathogens and water. Int J Env res Public Health. 2010;7:3657–703.
Batt CA. Food pathogen detection. Science. 2007;316:1579.
Nugen SR, Baeumner AJ. Trends and opportunities in food pathogen detection. Anal Bioanal Chem. 2008;391:451–4.
Cheung PY, Kwok KK, Kam KM. Application of BAX system, Tecra Unique Salmonella test, and a conventional culture method for the detection of Salmonella in ready-to-eat and raw foods. J Appl Microbiol. 2007;103:219–27.
Leonard P, Hearty S, Brennan J, Dunne L, Quinn J, Chakraborty T, O’Kennedy R. Advances in biosensors for detection of pathogens in food and water. Enzym Microb Technol. 2003;32:3–13.
Mandal P, Biswas A, Choi K, Pal U. Methods for rapid detection of foodborne pathogens: an overview. Am J Food Technol. 2011;6:87–102.
Mayrhofer S, Paulsen P, Smulders FJM, Hilbert F. Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultry. Int J Food Microbiol. 2004;97:23–9.
Rousseaux S, Hartmann A, Soulas G. Isolation and characterisation of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol Ecol. 2001;36:211–22.
Weinstein MP. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol. 2003;41:2275–8.
Duncan AW, Maggi RG, Breitschwerdt EB. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods. 2007;69:273–81.
Pérez FG, Mascini M, Tothill IE, Turner AP. Immunomagnetic separation with mediated flow injection analysis amperometric detection of viable Escherichia coli O157. Anal Chem. 1998;70:2380–6.
Gu H, Xu K, Xu C, Xu B. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun. 2006;9:941–9.
Zhu P, Shelton DR, Li S, Adams DL, Karns JS, Amstutz P, Tang CM. Detection of E. coli O157:H7 by immunomagnetic separation coupled with fluorescence immunoassay. Biosens Bioelectron. 2011;30:337–41.
Schwab KJ, De Leon R, Sobsey MD. Immunoaffinity concentration and purification of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl Environ Microbiol. 1996;62:2086–94.
Mannoor MS, Zhang S, Link AJ, McAlpine MC. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc Natl Acad Sci. 2010;107:19207–12.
Del Sesto RE, McCleskey TM, Burrell AK, Baker GA, Thompson JD, Scott BL, Wilkes JS, Williams P. Structure and magnetic behavior of transition metal based ionic liquids. Chem Commun. 2008;4:447–9.
Funasako Y, Mochida T, Inagaki T, Sakurai T, Ohta H, Furukawa K, Nakamura T. Magnetic memory based on magnetic alignment of a paramagnetic ionic liquid near room temperature. Chem Commun. 2011;47:4475–7.
Brown P, Butts CP, Eastoe J, Padron Hernandez E, Machado FLA, de Oliveira RJ. Dication magnetic ionic liquids with tuneable heteroanions. Chem Commun. 2013;49:2765–7.
Santos E, Albo J, Irabien A. Magnetic ionic liquids: synthesis, properties and applications. RSC Adv. 2014;4:40008–18.
Mester P, Wagner M, Rossmanith P. Use of ionic liquid-based extraction for recovery of Salmonella typhimurium and Listeria monocytogenes from food matrices. J Food Prot. 2010;73:680–7.
Ahmad F, Wu HF. Characterization of pathogenic bacteria using ionic liquid via single drop microextraction combined with MALDI-TOF MS. Analyst. 2011;136:4020–7.
Nacham O, Clark KD, Anderson JL. Analysis of bacterial plasmid DNA by solid-phase microextraction. Anal Methods. 2015;7:7202–7.
Rosatella AA, Siopa F, Frade RFM, Afonso CAM. New low viscous cholinium-based magnetic ionic liquids. New J Chem. 2016;40:3124–9.
Nacham O, Clark KD, Yu H, Anderson JL. Synthetic strategies for tailoring the physicochemical and magnetic properties of hydrophobic magnetic ionic liquids. Chem Mater. 2015;27:923–31.
Clark KD, Nacham O, Yu H, Li T, Yamsek MM, Ronning DR, Anderson JL. Extraction of DNA by magnetic ionic liquids: tunable solvents for rapid and selective DNA analysis. Anal Chem. 2015;87:1552–9.
Clark KD, Sorensen M, Nacham O, Anderson JL. Preservation of DNA in nuclease-rich samples using magnetic ionic liquids. RSC Adv. 2016;6:39846–51.
Clark KD, Yamsek MM, Nacham O, Anderson JL. Magnetic ionic liquids as PCR-compatible solvents for DNA extraction from biological samples. Chem Commun. 2015;51:16771–3.
Pierson SA, Nacham O, Clark KD, Nan H, Mudruk Y, Anderson JL. Synthesis and characterization of low viscosity hexafluoroacetylacetonate-based hydrophobic magnetic ionic liquids. New J Chem. 2017. doi:10.1039/C7NJ00206H.
Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–80.
Chapman P, Ellin M, Ashton R, Shafique W. Comparison of culture, PCR and immunoassays for detecting Escherichia coli O157 following enrichment culture and immunomagnetic separation performed on naturally contaminated raw meat products. Int J Food Microbiol. 2001;68:11–20.
Heininger A, Binder M, Schmidt S, Unertl K, Botzenhart K, Döring G. PCR and blood culture for detection of Escherichia coli bacteremia in rats. J Clin Microbiol. 1999;37:2479–82.
Acknowledgements
J.L.A. acknowledges funding from the Chemical Measurement and Imaging Program at the National Science Foundation (CHE-1413199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 281 kb).
Rights and permissions
About this article
Cite this article
Clark, K.D., Purslow, J.A., Pierson, S.A. et al. Rapid preconcentration of viable bacteria using magnetic ionic liquids for PCR amplification and culture-based diagnostics. Anal Bioanal Chem 409, 4983–4991 (2017). https://doi.org/10.1007/s00216-017-0439-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0439-y