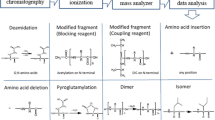
Abstract
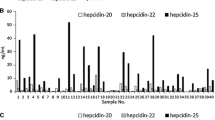
In metrology institutes, the state-of-the-art for purity analysis of peptides/proteins mainly addresses short and unfolded peptides. Important developments are anticipated for the characterization of nonlinear peptides or proteins. Hepcidin 1-25 is an interesting model system because this small protein contains four disulfide bridges with a particular connectivity that is difficult to reproduce and could induce a bias in quantification. Hepcidin 1-25 is involved in iron-related disorders and anemia, in an inflammatory context, and its clinical relevance in neurodegenerative disorders is under investigation. It is also an emerging biomarker. Recent inter-laboratory studies showed a need for standardization of hepcidin assay and the need to produce certified reference materials. This paper discusses two hepcidin standards from different synthesis pathways that have been characterized by high-resolution mass spectrometry and ion mobility mass spectrometry.





Similar content being viewed by others
Abbreviations
- hrMS:
-
High-resolution mass spectrometry
- IM-MS:
-
Ion mobility mass spectrometry
References
Lehmann S, Hoofnagle A, Hochstrasser D, Brede C, Glueckmann M, Cocho JA, Ceglarek U, Lenz C, Vialaret J, Scherl A, Hirtz C. Quantitative clinical chemistry proteomics (qCCP) using mass spectrometry: general characteristics and application. Clin Chem Lab Med. 2012; 1–16
Kroot JJC, Kemna EHJM, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94(12):1748–52.
Kroot JJC, van Herwaarden AE, Tjalsma H, Jansen RTP, Hendriks JCM, Swinkels DW. Second round robin for plasma hepcidin methods: first steps toward harmonization. Am J Hematol. 2012;87(10):977–83.
Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on In Vitro Diagnostic Medical Device, O.J.E.C.L.
Stoppacher N, Josephs RD, Daireaux A, Westwood S, Wielgosz RI. Impurity identification and determination for the peptide hormone angiotensin I by liquid chromatography-high-resolution tandem mass spectrometry and the metrological impact on value assignments by amino acid analysis. Anal Bioanal Chem. 2013;405(25):8039–51.
Stoppacher N, Josephs RD, Daireaux A, Choteau T, Westwood S, Wielgosz RI. Accurate quantification of impurities in pure peptide material-angiotensin I: comparison of calibration requirements and method performance characteristics of liquid chromatography coupled to hybrid tandem mass spectrometry and linear ion trap high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2015;29(18):1651–60.
Arsene CG, Henrion A, Diekmann N, Manolopoulou J, Bidlingmaier M. Quantification of growth hormone in serum by isotope dilution mass spectrometry. Anal Biochem. 2010;401(2):228–35.
Wu L, Takatsu A, Park S-R, Yang B, Yang H, Kinumi T, et al. Development and co-validation of porcine insulin certified reference material by high-performance liquid chromatography-isotope dilution mass spectrometry. Anal Bioanal Chem. 2015;407(11):3125–35.
Hare D, Ayton S, Bush A, Lei P. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5(34):1–19.
Connor JR, Ponnuru P, Wang XS, Patton SM, Allen RP, Earley CJ. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134(Pt 4):959–68.
Jordan JB, Poppe L, Haniu M, Arvedson T, Syed R, Li V, et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J Biol Chem. 2009;284(36):24155–67.
van der Vorm LN, Hendriks JC, Laarakkers CM, Klaver S, Armitage AE, Bamberg A, et al. Toward worldwide hepcidin assay harmonization: identification of a commutable secondary reference material. Clin Chem. 2016;62(7):993–1001.
Westwood S, Choteau T, Daireaux A, Josephs RD, Wielgosz RI. Mass balance method for the SI value assignment of the purity of organic compounds. Anal Chem. 2013;85(6):3118–26.
Josephs RD, Koeber R, Linsinger TP, Bernreuther A, Ulberth F, Schimmel H. Production of certified reference materials for mycotoxins: IRMM’s view on the assessment of uncertainties. Anal Bioanal Chem. 2004;378(5):1190–7.
Acknowledgements
The authors would like to thank Maria Colombo from Eurogentec for making available different batches of hepcidin material and for her support of the study design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted with synthetic hepcidin materials provided by Eurogentec (Seraing, Belgium).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bros, P., Josephs, R.D., Stoppacher, N. et al. Impurity determination for hepcidin by liquid chromatography-high resolution and ion mobility mass spectrometry for the value assignment of candidate primary calibrators. Anal Bioanal Chem 409, 2559–2567 (2017). https://doi.org/10.1007/s00216-017-0202-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0202-4