Abstract
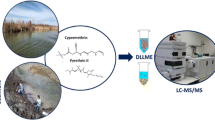
A rapid extraction procedure is presented for the determination of five endocrine-disrupting compounds, estrone, ethinylestradiol, bisphenol A, triclosan, and 2-ethylhexylsalicylate, in water samples. The analysis involves a two-step extraction procedure that combines dispersive liquid–liquid microextraction (DLLME) with dispersive micro-solid phase extraction (D-μ-SPE), using magnetic nanoparticles, followed by in situ derivatization in the injection port of a gas chromatograph coupled to triple quadrupole mass spectrometry. The use of uncoated or oleate-coated Fe3O4 nanoparticles as sorbent in the extraction process was evaluated and compared. The main parameters involved in the extraction process were optimized applying experimental designs. Uncoated Fe3O4 nanoparticles were selected in order to simplify and make more cost-effective the procedure. DLLME was carried out at pH 3, during 2 min, followed by the addition of the nanoparticles for D-μ-SPE employing 1 min in the extraction. Analysis of spiked water samples of different sources gave satisfactory recovery results for all the compounds with detection limits ranging from 7 to 180 ng l−1. Finally, the procedure was applied in tap, well, and river water.

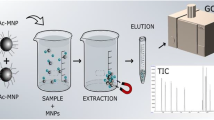
Diagram of the extraction method using magnetic nanoparticles (MNPs)


Similar content being viewed by others
References
WHO/IPCS (World Health Organization/International Petroleum Chemical Society). In: Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G, editors. Global assessment of the state-of-the-science of endocrine disruptors. WHO/IPCS/EDC/02.2. Geneva: World Health Organization; 2002. http://ehp.niehs.nih.gov/who/.
Snow DD, Damon-Powell T, Onanong S, Cassada DA. Sensitive and simplified analysis of natural and synthetic steroids in water and solids using on-line solid-phase extraction and microwave-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry atmospheric pressure photoionization. Anal Bioanal Chem. 2013;405:1759–71.
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342.
Gorga M, Insa S, Petrovic M, Barceló D. Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci Total Environ. 2015;503:69–86.
Ingerslev F, Halling-Sorensen B. Evaluation of analytical methods for detection of estrogens in the environment. Working Report No 44. Copenhagen: Danish Environmental Protection Agency; 2003.
Sosa-Ferrera Z, Mahugo-Santana C, Juan Santana-Rodriguez J. Analytical methodologies for the determination of endocrine disrupting compounds in biological and environmental samples. Biomed Res Int. 2013;Article ID 674838, 23 pages, doi:10.1155/2013/674838.
European Commission Endocrine Disruptors priority lists Annex 13. List of 146 substances with endocrine disruption classifications prepared in the Expert meeting http://ec.europa.eu/environment/archives/docum/pdf/bkh_annex_13.pdf
Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254:179–86.
European Commission COM/2011/0876 final—2011/0429 (COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy.
Sobek A, Bejgarn S, Ruden C, Molander L, Breitholtz M. In the shadow of the Cosmetic Directive—inconsistencies in EU environmental hazard classification requirements for UV-filters. Sci Total Environ. 2013;461:706–11.
Tomsikova H, Aufartova J, Solich P, Sosa-Ferrera Z, Santana-Rodriguez J, Novakova L. High-sensitivity analysis of female-steroid hormones in environmental samples. TrAC-Trends Anal Chem. 2012;34:35–58.
Pacakova V, Loukotkova L, Bosakova Z, Stulik K. Analysis for estrogens as environmental pollutants—a review. J Sep Sci. 2009;32:867–82.
Li Q, Wang X, Yuan D. Preparation of solid-phase microextraction fiber coated with single-walled carbon nanotubes by electrophoretic deposition and its application in extracting phenols from aqueous samples. J Chromatogr A. 2009;1216:1305–11.
Canosa P, Rodriguez I, Rubi E, Cela R. Optimization of solid-phase microextraction conditions for the determination of triclosan and possible related compounds in water samples. J Chromatogr A. 2005;1072:107–15.
Xie L, Jiang R, Zhu F, Liu H, Ouyang G. Application of functionalized magnetic nanoparticles in sample preparation. Anal Bioanal Chem. 2014;406:377–99.
Alcudia-Leon MC, Lucena R, Cardenas S, Valcarcel M. Magnetically confined hydrophobic nanoparticles for the microextraction of endocrine-disrupting phenols from environmental waters. Anal Bioanal Chem. 2013;405:2729–34.
Roman IP, Chisvert A, Canals A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography–mass spectrometry for UV-filter determination in water samples. J Chromatogr A. 2011;1218:2467–75.
Zhao XL, Shi YL, Ca YQ, Mou SF. Cetyltrimethylammonium bromide-coated magnetic nanoparticles for the preconcentration of phenolic compounds from environmental water samples. Environ Sci Technol. 2008;42:1201–6.
Jiang X, Cheng J, Zhou H, Li F, Wu W, Ding K. Polyaniline-coated chitosan-functionalized magnetic nanoparticles: preparation for the extraction and analysis of endocrine-disrupting phenols in environmental water and juice samples. Talanta. 2015;141:239–46.
Liu Y, Jia L. Analysis of estrogens in water by magnetic octadecylsilane particles extraction and sweeping micellar electrokinetic chromatography. Microchem J. 2008;89:72–6.
Ye L, Wang Q, Xu J, Shi ZG, Xu L. Restricted-access nanoparticles for magnetic solid-phase extraction of steroid hormones from environmental and biological samples. J Chromatogr A. 2012;1244:46–54.
Liu L, Feng T, Wang C, Wu Q, Wang Z. Magnetic three-dimensional graphene nanoparticles for the preconcentration of endocrine-disrupting phenols. Microchim Acta. 2014;181:1249–55.
Li F, Cai C, Cheng J, Zhou H, Ding K, Zhang L. Extraction of endocrine disrupting phenols with iron-ferric oxide core-shell nanowires on graphene oxide nanosheets, followed by their determination by HPLC. Microchim Acta. 2015;182:2503–11.
Tay KS, Abd Rahman N, Bin Abas MR. Magnetic nanoparticle assisted dispersive liquid-liquid microextraction for the determination of 4-n-nonylphenol in water. Anal Methods. 2013;5:2933–8.
Shi ZG, Lee HK. Dispersive liquid-liquid microextraction coupled with dispersive μ-solid-phase extraction for the fast determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Chem. 2010;82:1540–5.
Mukdasai S, Thomas C, Srijaranai S. Enhancement of sensitivity for the spectrophotometric determination of carbaryl using dispersive liquid microextraction combined with dispersive μ-solid phase extraction. Anal Methods. 2013;5:789–96.
Wang N, Shen R, Yan Z, Feng H, Cai Q, Yao S. Magnetic retrieval of an extractant: fast ultrasound assisted emulsification liquid-liquid microextraction for the determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Methods. 2013;5:3999–4004.
Pérez RA, Albero B, Tadeo JL, Sánchez-Brunete C. Oleate functionalized magnetic nanoparticles as sorbent for the analysis of polychlorinated biphenyls in juices. Microchim Acta. 2016;183:157–65.
Esteban S, Gorga M, Gonzalez-Alonso S, Petrovic M, Barceló D, Valcarcel Y. Monitoring endocrine disrupting compounds and estrogenic activity in tap water from Central Spain. Environ Sci Pollut R. 2014;21:9297–310.
Gorga M, Petrovic M, Barceló D. Multi-residue analytical method for the determination of endocrine disruptors and related compounds in river and waste water using dual column liquid chromatography switching system coupled to mass spectrometry. J Chromatogr A. 2013;1295:57–66.
Esteban S, Gorga M, Petrovic M, Gonzalez-Alonso S, Barceló D, Valcarcel Y. Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci Total Environ. 2014;466:939–51.
Carvalho ARM, Cardoso VV, Rodrigues A, Ferreira E, Benoliel MJ, Duarte EA. Quality assessment of sulfurous thermal waters in the city of Poços de Caldas, Minas Gerais, Brazil. Environ Monit Assess. 2015;187(139):1–8.
Martin J, Santos JL, Aparicio I, Alonso E. Determination of hormones, a plasticizer, preservatives, perfluoroalkylated compounds, and a flame retardant in water samples by ultrasound-assisted dispersive liquid-liquid microextraction based on the solidification of a floating organic drop. Talanta. 2015;143:335–43.
Azzouz A, Ballesteros E. Trace analysis of endocrine disrupting compounds in environmental water samples by use of solid-phase extraction and gas chromatography with mass spectrometry detection. J Chromatogr A. 2014;1360:248–57.
Zhang ZL, Hibberd A, Zhou JL. Optimisation of derivatisation for the analysis of estrogenic compounds in water by solid-phase extraction gas chromatography-mass spectrometry. Anal Chim Acta. 2006;577:52–61.
Mudiam MKR, Jain R, Singh R. Application of ultrasound-assisted dispersive liquid-liquid microextraction and automated in-port silylation for the simultaneous determination of phenolic endocrine disruptor chemicals in water samples by gas chromatography-triple quadrupole mass spectrometry. Anal Methods. 2014;6:1802–10.
Albero B, Sánchez-Brunete C, Miguel E, Perez RA, Tadeo JL. Determination of selected organic contaminants in soil by pressurized liquid extraction and gas chromatography tandem mass spectrometry with in situ derivatization. J Chromatogr A. 2012;1248:9–17.
Hibberd A, Maskaoui K, Zhang Z, Zhou JL. An improved method for the simultaneous analysis of phenolic and steroidal estrogens in water and sediment. Talanta. 2009;77:1315–21.
Albero B, Sánchez-Brunete C, Miguel E, Pérez RA, Tadeo JL. Analysis of natural-occurring and synthetic sexual hormones in sludge-amended soils by matrix solid-phase dispersion and isotope dilution gas chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1283:39–45.
Pérez RA, Albero B, Tadeo JL, Molero E, Sánchez-Brunete C. Application of magnetic iron oxide nanoparticles for the analysis of PCBs in water and soil leachates by gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407:1913–24.
Zhang L, He R, Gu H-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci. 2006;253:2611–7.
Li Y, Yang X, Zhang J, Li M, Zhao X, Yuan K, et al. Ultrasound-assisted emulsification magnetic microextraction: a fast and green method for the determination of triazole fungicides in fruit juice. Anal Methods. 2014;6:8328–36.
Benedé JL, Chisvert A, Salvador A, Sánchez-Quiles D, Tovar-Sánchez A. Determination of UV filters in both soluble and particulate fractions of seawaters by dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Anal Chim Acta. 2014;812:50–8.
Celano R, Piccinelli AL, Campone L, Rastrelli L. Ultra-preconcentration and determination of selected pharmaceutical and personal care products in different water matrices by solid-phase extraction combined with dispersive liquid–liquid microextraction prior to ultra high pressure liquid chromatography tandem mass spectrometry analysis. J Chromatogr A. 2014;1355:26–35.
Cunha SC, Pena A, Fernandes JO. Dispersive liquid-liquid microextraction followed by microwave-assisted silylation and gas chromatography-mass spectrometry analysis for simultaneous trace quantification of bisphenol A and 13 ultraviolet filters in wastewaters. J Chromatogr A. 2015;1414:10–21.
Zhang YF, Lee HK. Ionic liquid-based ultrasound-assisted dispersive liquid–liquid microextraction followed high-performance liquid chromatography for the determination of ultraviolet filters in environmental water samples. Anal Chim Acta. 2012;750:120–6.
Bernal E (2014) Limit of detection and limit of quantification determination in gas chromatography. In: Guo X, editor. Advances in gas chromatography. Intech, 2014, doi: 10.5772/57341
Chang C-C, Huang S-D. Determination of the steroid hormone levels in water samples by dispersive liquid-liquid microextraction with solidification of a floating organic drop followed by high-performance liquid chromatography. Anal Chim Acta. 2010;662:39–43.
Meng J, Shi C, Wei B, Yu W, Deng C, Zhang X. Preparation of Fe3O4@C@PANI magnetic microspheres for the extraction and analysis of phenolic compounds in water samples by gas chromatography-mass spectrometry. J Chromatogr A. 2011;1218:2841–7.
Pérez RA, Albero B, Tadeo JL, Molero E, Sánchez-Brunete C. Analysis of steroid hormones in water using palmitate-coated magnetite nanoparticles solid-phase extraction and gas chromatography-tandem mass spectrometry. Chromatographia. 2014;77:837–43.
Wu JW, Chen HC, Ding WH. Ultrasound-assisted dispersive liquid–liquid microextraction plus simultaneous silylation for rapid determination of salicylate and benzophenone-type ultraviolet filters in aqueous samples. J Chromatogr A. 2013;1302:20–7.
Zheng C, Zhao J, Bao P, Gao J, He J. Dispersive liquid-liquid microextraction based on solidification of floating organic droplet followed by high-performance liquid chromatography with ultraviolet detection and liquid chromatography–tandem mass spectrometry for the determination of triclosan and 2,4-dichlorophenol in water samples. J Chromatogr A. 2011;1218:3830–6.
Acknowledgments
This study was financed by the Ministry of Science and Innovation–National Institute for Agricultural and Food Research and Technology, INIA, Project number RTA2014-00012-C03-01. The authors wish to express their gratefulness to Dr. M.P. Morales, researcher of the Department of Biomaterials and Bioinspired Materials (ICMM; CSIC), for her help and advice in the characterization of the MNPs and to the microanalysis laboratory of the Institute of General Organic Chemistry (CSIC) for the elemental analysis of the MNPs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pérez, R.A., Albero, B., Tadeo, J.L. et al. Determination of endocrine-disrupting compounds in water samples by magnetic nanoparticle-assisted dispersive liquid–liquid microextraction combined with gas chromatography–tandem mass spectrometry. Anal Bioanal Chem 408, 8013–8023 (2016). https://doi.org/10.1007/s00216-016-9899-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9899-8