Abstract
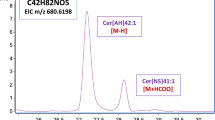
Skin, the largest organ of the human body, serves as the primary barrier to the external environment. Ceramides are one of the main constituents of stratum corneum (SC), playing an important role in skin barrier function. Therefore, comprehensive profiling and quantification of SC ceramide is important. Herein, a new targeted lipidomic method for human SC ceramide profiling and quantification is presented and tested. Normal-phase high-performance liquid chromatography coupled with dynamic multiple reaction monitoring mass spectrometry (NP-HPLC-dMRM-MS) was used to separate ceramides into subclasses and then characterize different ceramides within each subclass on the basis of their characteristics. In total, 483 ceramides were quantified in a single run within 20 min, covering 12 subclasses as well as some glycosylated ceramides not previously reported. Each subclass had typical standard substances (if available) that served to establish representative standard curves and were used for related substances with no standards. Linearity range, limit of quantification (LOQ), limit of detection (LOD), precision, accuracy, stability, and matrix effects were validated. dMRM increased sensitivity and accuracy greatly compared with common MRM (cMRM). This method was successfully applied to the study of human SC from different age groups. A total of 193 potential biomarkers were found to indicate age differences between children and adults. This method is an innovative approach for high-throughput quantification of SC ceramide.

Method establishment (MRM spectra by the established method) and method application (score scatter plots of authentic samples)





Similar content being viewed by others
Abbreviations
- APCI:
-
Atmospheric pressure chemical ionization
- CE:
-
Collision energy
- cMRM:
-
Common MRM
- dMRM-MS:
-
Dynamic multiple reaction monitoring mass spectrometry
- ESI:
-
Electrospray ionization
- IS:
-
Internal standard
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- NP-HPLC:
-
Normal-phase high-performance liquid chromatography
- QC:
-
Quality control
- RSD:
-
Relative standard deviation
- RT:
-
Retention time
- SC:
-
Stratum corneum
- SIM:
-
Selected ion monitoring
- SPE:
-
Solid-phase extraction
- TIC:
-
Total ion chromatography
- TLC:
-
Thin-layer chromatography
References
Kendall AC, Nicolaou A. Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res. 2013;52:141–64.
T’Kindt R, Jorge L, Dumont E, Couturon P, David F, Sandra P, et al. Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem. 2012;84:403–11.
Qu F, Wu CS, Hou JF, Jin Y, Zhang JL. Sphingolipids as new biomarkers for assessment of delayed-type hypersensitivity and response to triptolide. PLoS ONE. 2012;7:5806–19.
Merrill Jr AH, Stokes TH, Momin A, Park H, Portz BJ, Kelly S, et al. Sphingolipidomics: a valuable tool for understanding the roles of sphingolipids in biology and disease. J Lipid Res. 2009;50:S97–102.
Lew BL, Cho Y, Kim J, Sim WY, Kim NI. Ceramides and cell signaling molecules in psoriatic epidermis: reduced levels of ceramides, PKC-a, and JNK. J Korean Med Sci. 2006;21:95–9.
Hong KK, Cho HR, Ju WC, Cho Y, Kim NI. A study on altered expression of serine palmitoyltransferase and ceramidase in psoriatic skin lesion. J Korean Med Sci. 2007;22:862–7.
Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–66.
Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511–4.
Pavicic T, Wollenweber U, Farwick M, Korting HC. Anti-microbial and inflammatory activity and efficacy of phytosphingosine: an in vitro and in vivo study addressing acne vulgaris. Int J Cosmet Sci. 2007;29:181–90.
Bak H, Hong SP, Jeong SK, Choi EH, Lee SE, Lee SH, et al. Altered epidermal lipid layers induced by long-term exposure to suberythemal-dose ultraviolet. Int J Dermatol. 2011;50:832–7.
Pettus BJ, Baes M, Busman M, Hannun YA, Van Veldhoven PP. Mass spectrometric analysis of ceramide perturbations in brain and fibroblasts of mice and human patients with peroxisomal disorders. Rapid Commun Mass Spectrom. 2004;18:1569–74.
Han X. Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;302:199–212.
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, et al. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–76.
Van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52:1211–21.
Imokawa G, Akasaki S, Hattori M, Yoshizuka N. Selective recovery of deranged water-holding properties by stratum corneum lipids. J Invest Dermatol. 1986;87:758–61.
Holleran WM, Feingold KR, Man MQ, Gao WN, Lee JM, Elias PM. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991;32:1151–8.
Houjou T, Yamatani K, Imagawa M, Shimizu T, Taguchi R. A shotgun tandem mass spectrometric analysis of phospholipids with normal-phase and/or reverse-phase liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:654–66.
Masukawa Y, Narita H, Sato H, Naoe A, Kondo N, Sugai Y, et al. Comprehensive quantification of ceramide species in human stratum corneum. J Lipid Res. 2009;50:1708–19.
Farwanah H, Nuhn P, Neubert R, Raith K. Normal-phase liquid chromatographic separation of stratum corneum ceramides with detection by evaporative light scattering and atmospheric pressure chemical ionization mass spectrometry. Anal Chim Acta. 2003;492:233–9.
Farwanah H, Pierstorff B, Schmelzer CE, Raith K, Neubert RH, Kolter T, et al. Separation and mass spectrometric characterization of covalently bound skin ceramides using LC/APCI-MS and Nano-ESI-MS/MS. J Chromatogr B. 2007;852:562–70.
Dong Y, Yan K, Ma Y, Wang S, He G, Deng J, et al. A sensitive dilute-and-shoot approach for the simultaneous screening of 71 stimulants and 7 metabolites in human urine by LC-MS-MS with dynamic MRM. J Chromatogr Sci. 2015;53:1528–35.
Li J, Hu C, Zhao X, Dai W, Chen S, Lu X, et al. Large-scaled human serum sphingolipid profiling by using reversed-phase liquid chromatography coupled with dynamic multiple reaction monitoring of mass spectrometry: method development and application in hepatocellular carcinoma. J Chromatogr A. 2013;1320:103–10.
Farwanah H, Raith K, Neubert RH, Wohlrab J. Ceramide profiles of the uninvolved skin in atopic dermatitis and psoriasis are comparable to those of healthy skin. Arch Dermatol Res. 2005;296:514–21.
Higuchi H, Nakamura M, Kuwano A, Kasamatsu M, Nagahata H. Quantities and types of ceramides and their relationships to physical properties of the horn covering the claws of clinically normal cows and cows with subclinical laminitis. Can J Vet Res. 2005;69:155–8.
Farwanah H, Wohlrab J, Neubert RH, Raith K. Profiling of human stratum corneum ceramides by means of normal phase LC/APCI–MS. Anal Bioanal Chem. 2005;383:632–7.
Van Smeden J, Boiten WA, Hankemeier T, Rissmann R, Bouwstra JA, Vreeken RJ. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim Biophys Acta. 1841;2014:70–9.
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–51.
Robson KJ, Stewart ME, Michelsen S, Lazo ND, Downing DT. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J Lipid Res. 1994;35:2060–8.
USFDA. Guidance for industry: bioanalytical method validation. Federal Register. 2001;66:206–207.
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85.
Bao Y, Zhao T, Wang X, Qiu Y, Su M, Jia W, et al. Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res. 2009;8:1623–30.
Almeida R, Pauling JK, Sokol E, Hannibal-Bach HK, Ejsing CS. Comprehensive lipidome analysis by shotgun lipidomics on a hybrid quadrupole-orbitrap-linear ion trap mass spectrometer. J Am Soc Mass Spectrom. 2015;26:476–91.
Yoo HH, Son J, Kim DH. Liquid chromatography–tandem mass spectrometric determination of ceramides and related lipid species in cellular extracts. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:327–33.
Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91.
Scherer M, Leuthäuser-Jaschinski K, Ecker J, Schmitz G, Liebisch G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J Lipid Res. 2010;51:2001–11.
Kasumov T, Huang H, Chung YM, Zhang R, McCullough AJ, Kirwan JP. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2010;401:154–61.
Miyauchi Y. Make an observation about neonatal and infantile skins: how does the skin continue to develop and reach maturity? J Jpn Cosmet Sci Soc. 2014;38:28–36.
Tang N. High throughput protein quantitation using MRM viewer software and dynamic MRM on a triple quadruple mass spectrometer. J Biomol Tech. 2010;21:S60.
Ji ES, Cheon MH, Lee JY, Yoo JS, Jung HJ, Kim JY. Dynamic MRM measurements of multi-biomarker proteins by triple-quadrupole mass spectrometry with nano flow HPLC-microfluidics chip. Mass Spectrom Lett. 2010;1:21–4.
Yan Z, Li T, Lv P, Li X, Zhou C, Yang X. Sensitive and reliable multianalyte quantitation of herbal medicine in rat plasma using dynamic triggered multiple reaction monitoring. J Chromatogr B. 2013;928:22–31.
Liang J, Fu J, Todorovska MI, Trifunac MD. Effects of site dynamic characteristics on soil–structure interaction (II): incident P and SV waves. Soil Dyn Earthq Eng. 2013;51:58–76.
Acknowledgments
This work was supported by grants of the Natural Science Foundation of Beijing Municipality (7154197) and grants of the National Health and Family Planning Commission of the People’s Republic of China (201402001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were reviewed and approved by the Ethics Committee of Beijing Children’s Hospital and the methods were carried out in accordance with the Declaration of Helsinki.
Conflict of interest
The authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 789 kb)
Rights and permissions
About this article
Cite this article
Jia, ZX., Zhang, JL., Shen, CP. et al. Profile and quantification of human stratum corneum ceramides by normal-phase liquid chromatography coupled with dynamic multiple reaction monitoring of mass spectrometry: development of targeted lipidomic method and application to human stratum corneum of different age groups. Anal Bioanal Chem 408, 6623–6636 (2016). https://doi.org/10.1007/s00216-016-9775-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9775-6