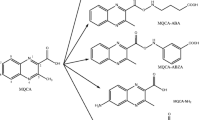
Abstract
Microcystins (MCs) and nodularin (NOD) are cyanobacterial hepatotoxins that can greatly harm human health. Multi-analyte immunoassays provide efficient and cheap methods of screening these toxins. To develop a multi-analyte immunoassay, an antibody with both broad specificity and high affinity for structurally similar algal toxins is urgently needed. In this study, microcystin–leucine–arginine (MC-LR) and NOD were conjugated to carrier proteins using a one-step active ester (AE) method and multistep thiol-ene click chemistry and glutaraldehyde method, respectively. The immunogens obtained from these two conjugation methods were evaluated for their effectiveness in producing antibodies. The results demonstrated that the antisera derived from AE immunogens showed better performance in terms of affinity and titer. Using this simple AE method, we prepared a new immunogen for NOD and successfully produced a monoclonal antibody (mAb), 2G5, which could recognize not only NOD but also all eight of the tested MCs (MC-LR, MC-RR, MC-YR, MC-WR, MC-LA, MC-LF, MC-LY, and MC-LW) with high sensitivity and improved uniform affinities (0.23 ≤ IC50 ≤ 0.68 ng mL−1) compared with previously described mAbs. Under optimal conditions, one indirect competitive enzyme-linked immunosorbent assay was developed based on mAb2G5 for the detection of MC-LR and NOD, with limits of detection of 0.16 and 0.10 μg L−1, respectively, and a recovery of 62–86 % with a coefficient of variation below 12.6 % in water samples.



Similar content being viewed by others
References
Humpage A. Toxin types, toxicokinetics and toxicodynamics. Adv Exp Med Biol. 2008;619:383–415.
Mackintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–92. doi:10.1016/0014-5793(90)80245-E.
Rinehart KL, Harada K, Namikoshi M, et al. Nodularin, microcystin, and the configuration of Adda. J Am Chem Soc. 1988;110:8557–8. doi:10.1021/ja00233a049.
Rinehart KL, Namikoshi M, Choi BW. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J Appl Phycol. 1994;6:159–76. doi:10.1007/BF02186070.
Spoof L, Vesterkvist P, Lindholm T, Meriluoto J. Screening for cyanobacterial hepatotoxins, microcystins and nodularin in environmental water samples by reversed-phase liquid chromatography-electrospray ionisation mass spectrometry. J Chromatogr A. 2003;1020:105–19. doi:10.1016/S0021-9673(03)00428-X.
Carmichael WW. The toxins of cyanobacteria. Sci Am. 1994;270:78–86. doi:10.1038/scientificamerican0194-78.
Rastogi RP, Sinha RP, Incharoensakdi A. The cyanotoxin-microcystins: current overview. Rev Environ Sci Biol. 2014;13:215–49. doi:10.1007/s11157-014-9334-6.
World Health Organization. Algae and cyanobacteria in fresh water. In: Guidelines for safe recreational water environments. Geneva, Switzerland: WHO; 2003. p. 136–58.
World Health Organization. Cyanobacterial toxins: microcystin-LR. In: Guidelines for drinking water quality. Geneva, Switzerland: WHO; 1998. p. 95–110.
Nagata S, Tsutsumi T, Hasegawa A, Yoshida F, Ueno Y, Watanabe MF. Enzyme immunoassay for direct determination of microcystins in environmental water. J AOAC Int. 1997;80:408–17.
Rapala J, Erkomaa K, Kukkonen J, Sivonen K, Lahti K. Detection of microcystins with protein phosphatase inhibition assay, high-performance liquid chromatography-UV detection and enzyme-linked immunosorbent assay: comparison of methods. Anal Chim Acta. 2002;466:213–31. doi:10.1016/S0003-2670(02)00588-3.
Murphy C, Stack E, Krivelo S, et al. Detection of the cyanobacterial toxin, microcystin-LR, using a novel recombinant antibody-based optical-planar waveguide platform. Biosens Bioelectron. 2015;67:708–14. doi:10.1016/j.bios.2014.10.039.
Weller MG. Immunoassays and biosensors for the detection of cyanobacterial toxins in water. Sensors (Basel). 2013;13:15085–112. doi:10.3390/s131115085.
Devlin S, Meneely JP, Greer B, Campbell K, Vasconcelos V, Elliott CT. Production of a broad specificity antibody for the development and validation of an optical SPR screening method for free and intracellular microcystins and nodularin in cyanobacteria cultures. Talanta. 2014;122:8–15. doi:10.1016/j.talanta.2013.12.065.
Khreich N, Lamourette P, Renard PY, et al. A highly sensitive competitive enzyme immunoassay of broad specificity quantifying microcystins and nodularins in water samples. Toxicon. 2009;53:551–9. doi:10.1016/j.toxicon.2008.12.021.
Gurbuz F, Metcalf JS, Codd GA, Karahan AG. Evaluation of enzyme-linked immunosorbent assays (ELISAs) for the determination of microcystins in cyanobacteria. Environ Forensic. 2012;13:105–9. doi:10.1080/15275922.2012.676596.
Zhou Y, Li Y, Zhi B, et al. Detection of nodularin based on a monoclonal antibody in water and aquatic fish samples. Food Control. 2011;22:797–800. doi:10.1016/j.foodcont.2010.08.006.
Morenkov OS, Vrublevskaya VV, Kochkina NV, Kovtun AL. Development of an immunoenzyme method for detection and quantitative determination of microcystins. Inland Water Biol. 2014;7:299–305. doi:10.1134/S1995082914030146.
Lotierzo M, Abuknesha R, Davis F, Tothill IE. A membrane-based ELISA assay and electrochemical immunosensor for microcystin-LR in water samples. Environ Sci Technol. 2012;46:5504–10. doi:10.1021/es2041042.
Sheng JW, He M, Shi HC, Qian Y. A comprehensive immunoassay for the detection of microcystins in waters based on polyclonal antibodies. Anal Chim Acta. 2006;572:309–15. doi:10.1016/j.aca.2006.05.040.
Liu B, Yu F, Chu FS. Anti-idiotype and anti-anti-idiotype antibodies generated from polyclonal antibodies against microcystin-LR. J Agric Food Chem. 1996;44:4037–42. doi:10.1021/jf960286k.
Tsutsumi T, Nagata S, Yoshida F, Ueno Y. Anti-idiotype monoclonal antibodies against anti-microcystin antibody and their use in enzyme immunoassay. Toxicon. 1998;36:235–45. doi:10.1016/S0041-0101(97)00130-X.
Nagata S, Tsutsumi T, Yoshida F, Ueno Y. A new type sandwich immunoassay for microcystin: production of monoclonal antibodies specific to the immune complex formed by microcystin and an anti-microcystin monoclonal antibody. Nat Toxins. 1999;7:49–55. doi:10.1002/(SICI)1522-7189(199903/04)7:2<49::AID-NT43>3.0.CO;2-7.
Tsutsumi T, Nagata S, Yoshida F, Ueno Y, Harada KI. Development and application of highly sensitive anti-immune complex ELISAs for microcystins in tap water. Food Agric Immunol. 2000;12:231–41. doi:10.1080/09540100050140768.
McElhiney J, Drever M, Lawton LA, Porter AJ. Rapid isolation of a single-chain antibody against the cyanobacterial toxin microcystin-LR by phage display and its use in the immunoaffinity concentration of microcystins from water. Appl Environ Microbiol. 2002;68:5288–95. doi:10.1128/AEM.68.11.5288-5295.2002.
Zeck A, Weller MG, Bursill D, Niessner R. Generic microcystin immunoassay based on monoclonal antibodies against Adda. Analyst. 2001;126:2002–7. doi:10.1039/b105064h.
Harada K, Imanishi S, Kato H, Mizuno M, Ito E, Tsuji K. Isolation of Adda from microcystin-LR by microbial degradation. Toxicon. 2004;44:107–9. doi:10.1016/j.toxicon.2004.04.003.
Samdal IA, Ballot A, Løvberg KE, Miles CO. Multihapten approach leading to a sensitive ELISA with broad cross-reactivity to microcystins and nodularin. Environ Sci Technol. 2014;48:8035–43. doi:10.1021/es5012675.
Kfir R, Johannsen E, Botes DP. Monoclonal antibody specific for cyanoginosin-LA: preparation and characterization. Toxicon. 1986;24:543–52. doi:10.1016/0041-0101(86)90174-1.
Young FM, Metcalf JS, Meriluoto JA, Spoof L, Morrison LF, Codd GA. Production of antibodies against microcystin-RR for the assessment of purified microcystins and cyanobacterial environmental samples. Toxicon. 2006;48:295–306. doi:10.1016/j.toxicon.2006.05.015.
Fischer WJ, Garthwaite I, Miles CO, et al. Congener-independent immunoassay for microcystins and nodularins. Environ Sci Technol. 2001;35:4849–56. doi:10.1021/es011182f.
Mikhailov A, Härmälä-Braskén AS, Polosukhina E, et al. Production and specificity of monoclonal antibodies against nodularin conjugated through N-methyldehydrobutyrine. Toxicon. 2001;39:1453–9. doi:10.1016/S0041-0101(01)00104-0.
Yu FY, Liu BH, Chou HN, Chu FS. Development of a sensitive ELISA for the determination of microcystins in algae. J Agric Food Chem. 2002;50:4176–82. doi:10.1021/jf0202483.
Sheng JW, He M, Shi HC. A highly specific immunoassay for microcystin-LR detection based on a monoclonal antibody. Anal Chim Acta. 2007;603:111–8. doi:10.1016/j.aca.2007.09.029.
Wang Z, Li N, Zhang S, Zhang H, Sheng Y, Shen J. Production of antibodies and development of enzyme-linked immunosorbent assay for valnemulin in porcine liver. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:244–52. doi:10.1080/19440049.2012.738370.
Pyo D, Lee J, Choi E. Trace analysis of microcystins in water using enzyme-linked immunosorbent assay. Microchem J. 2005;80:165–9. doi:10.1016/j.microc.2004.07.015.
Mhadhbi H, Ben-Rejeb S, Cléroux C, Martel A, Delahaut P. Generation and characterization of polyclonal antibodies against microcystins—application to immunoassays and immunoaffinity sample preparation prior to analysis by liquid chromatography and UV detection. Talanta. 2006;70:225–35. doi:10.1016/j.talanta.2006.02.029.
Metcalf JS, Bell SG, Codd GA. Production of novel polyclonal antibodies against the cyanobacterial toxin microcystin-LR and their application for the detection and quantification of microcystins and nodularin. Water Res. 2000;34:2761–9. doi:10.1016/S0043-1354(99)00429-7.
Baier W, Loleit M, Fischer B, et al. Generation of antibodies directed against the low-immunogenic peptide-toxins microcystin-LR/RR and nodularin. Int J Immunopharmacol. 2000;22:339–53. doi:10.1016/S0192-0561(99)00086-7.
Mikhailov A, Härmälä-Braskén AS, Meriluoto J, Sorokina Y, Dietrich D, Eriksson JE. Production and specificity of mono and polyclonal antibodies against microcystins conjugated through N-methyldehydroalanine. Toxicon. 2001;39:477–83. doi:10.1016/S0041-0101(00)00148-3.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (U1301214).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Published in the topical collection Immunoanalysis for Environmental Monitoring and Human Health with guest editors Shirley J. Gee, Ivan R. Kennedy, Alice Lee, Hideo Ohkawa, Tippawan Prapamontol, and Ting Xu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 524 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Dai, R., Zhang, H. et al. Production of monoclonal antibodies with broad specificity and development of an immunoassay for microcystins and nodularin in water. Anal Bioanal Chem 408, 6037–6044 (2016). https://doi.org/10.1007/s00216-016-9692-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9692-8