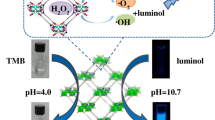
Abstract
Various analytical applications of metal–organic frameworks (MOFs) have been rapidly developed in the past few years. However, the employment of MOFs as catalysts in chemiluminescence (CL) analysis is rare. Here, for the first time, we found that MIL-53(Fe) MOFs could significantly enhance the CL of luminol in the presence of H2O2 in an alkaline medium. The CL intensity in the luminol–H2O2–MIL-53(Fe) system was about 20 times higher than that in the luminol–H2O2 system. Moreover, the XRD pattern of MIL-53(Fe) after CL reaction was almost the same as that of the original MIL-53(Fe), confirming the catalytic role of MIL-53(Fe) in the luminol–H2O2–MIL-53(Fe) system. The possible mechanism behind the enhancing phenomenon was discussed based on the results from the CL spectra, FL probe experiments, and active oxygen species measurements. By coupling with the glucose oxidase-based catalytic oxidation reaction, a sensitive and selective CL method was developed for the detection of glucose. There is a linear relationship between the logarithm of CL intensity and the logarithm of glucose concentration in the range from 0.1 to 10 μM, and a detection limit of 0.05 μM (S/N = 3) is obtained. The proposed method has been applied to the determination of glucose in human serum samples with satisfactory results.

MIL-53(Fe) MOFs are found to greatly enhance the chemiluminescence emission of the luminol–H2O2 system, and this finding resulted in a new chemiluminescence method for biosensing of glucose when coupled with the glucose oxidase.




Similar content being viewed by others
References
Chen H, Lin L, Li H, Li J, Lin JM. Aggregation-induced structure transition of protein-stabilized zinc/copper nanoclusters for amplified chemiluminescence. ACS Nano. 2015;9:2173–83.
Zhou W, Cao Y, Sui D, Lu C. Radical pair-driven luminescence of quantum dots for specific detection of peroxynitrite in living cells. Anal Chem. 2016;88:2659–65.
Shi W, Zhang X, He S, Huang Y. CoFe2O4 magnetic nanoparticles as peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem Commun. 2011;47:10785–7.
Zhang LJ, Chen YC, Zhang ZM, Lu C. Highly selective sensing of hydrogen peroxide based on cobalt–ethylenediaminetetraacetate complex intercalated layered double hydroxide-enhanced luminol chemiluminescence. Sensors Actuators B Chem. 2014;193:752–8.
Li N, Liu D, Cui H. Metal-nanoparticle-involved chemiluminescence and its applications in bioassays. Anal Bioanal Chem. 2014;406:5561–71.
He Y, He X, Liu X, Gao L, Cui H. Dynamically tunable chemiluminescence of luminol-functionalized silver nanoparticles and its application to protein sensing arrays. Anal Chem. 2014;86:12166–71.
He S, Shi W, Zhang X, Li J, Huang Y. β-cyclodextrins-based inclusion complexes of CoFe2O4 magnetic nanoparticles as catalyst for the luminol chemiluminescence system and their applications in hydrogen peroxide detection. Talanta. 2010;82:377–83.
Guan G, Yang L, Mei Q, Zhang K, Zhang Z, Han M. Chemiluminescence switching on peroxidase-like Fe3O4 nanoparticles for selective detection and simultaneous determination of various pesticides. Anal Chem. 2012;84:9492–7.
Dong S, Zhong J, Lu C. Introducing confinement effects into ultraweak chemiluminescence for an improved sensitivity. Anal Chem. 2014;86:7947–53.
Guo Y, Li B. Carbon dots-initiated luminol chemiluminescence in the absence of added oxidant. Carbon. 2015;82:459–69.
Dou X, Lin Z, Chen H, Zheng Y, Lu C, Lin JM. Production of superoxide anion radicals as evidence for carbon nanodots acting as electron donors by the chemiluminescence method. Chem Commun. 2013;49:5871–3.
Wang Z, Liu F, Teng X, Zhao C, Lu C. Detection of hydrogen peroxide in rainwater based on Mg-Al-carbonate layered double hydroxides-catalyzed luminol chemiluminescence. Analyst. 2011;136:4986–90.
Zhou W, Cao Y, Sui D, Lu C. Turn-on luminescent probes for the real-time monitoring of endogenous hydroxyl radicals in living cells. Angew Chem Int Ed. 2016;55:4236–41.
Tang Y, Su Y, Yang N, Zhang L, Lv Y. Carbon nitride quantum dots: a novel chemiluminescence system for selective detection of free chlorine in water. Anal Chem. 2014;86:4528–35.
Dong S, Liu F, Lu C. Organo-modified hydrotalcite-quantum dots nanocomposites as novel chemiluminescence resonance energy transfer probe. Anal Chem. 2013;85:3363–8.
Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. Metal–organic framework materials as chemical sensors. Chem Rev. 2012;112:1105–25.
Li J, Sculley J, Zhou H-C. Metal–organic frameworks for separations. Chem Rev. 2012;112:869–932.
Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Metal–organic framework materials as catalysts. Chem Soc Rev. 2009;38:1450–9.
Cui Y, Yue Y, Qian G, Chen B. Luminescent functional metal–organic frameworks. Chem Rev. 2012;112:1126–62.
Yang CX, Ren HB, Yan XP. Fluorescent metal–organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal Chem. 2013;85:7441–6.
Feng D, Gu ZY, Li JR, Jiang HL, Wei Z, Zhou HC. Zirconium-metalloporphyrin PCN-222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew Chem Int Ed. 2012;51:10307–10.
Ai L, Li L, Zhang C, Fu J, Jiang J. MIL-53(Fe): a metal–organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem Eur J. 2013;19:15105–8.
Zhang J, Zhang H, Du Z, Wang X, Yu S, Jiang H. Water-stable metal–organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem Commun. 2014;50:1092–4.
Zhu Q, Chen Y, Wang W, Zhang H, Ren C, Chen H, et al. A sensitive biosensor for dopamine determination based on the unique catalytic chemiluminescence of metal–organic framework HKUST-1. Sensors Actuators B Chem. 2015;210:500–7.
Yang N, Song H, Wan X, Fan X, Su Y, Lv Y. A metal (Co)-organic framework-based chemiluminescence system for selective detection of L-cysteine. Analyst. 2015;140:2656–63.
Luo F, Lin Y, Zheng L, Lin X, Chi Y. Encapsulation of Hemin in metal–organic frameworks for catalyzing the chemiluminescence reaction of the H2O2–luminol system and detecting glucose in the neutral condition. ACS Appl Mater Interfaces. 2015;7:11322–9.
Deng H, Wu G, He D, Peng H, Liu A, Xia X, et al. Fenton reaction-mediated fluorescence quenching of N-acetyl-L-cysteine-protected gold nanoclusters: analytical applications of hydrogen peroxide, glucose, and catalase detection. Analyst. 2015;140:7650–6.
Han L, Zeng L, Wei M, Lie CM, Liu A. A V2O3-ordered mesoporous carbon composite with novel peroxidase-like activity towards the glucose colorimetric assay. Nanoscale. 2015;7:11678–85.
Khajvand T, Alijanpour O, Chaichi MJ, Vafaeezadeh M, Hashemi MM. Imidazolium-based ionic liquid derivative/CuII complexes as efficient catalysts of the lucigenin chemiluminescence system and its application to H2O2 and glucose detection. Anal Bioanal Chem. 2015;407:6127–36.
Sato T, Katayama K, Arai T, Sako T, Tazaki H. Simultaneous determination of serum mannose and glucose concentrations in dog serum using high performance liquid chromatography. Res Vet Sci. 2008;84:26–9.
Wang Y, Li H, Kong J. Facile preparation of mesocellular graphene foam for direct glucose oxidase electrochemistry and sensitive glucose sensing. Sensors Actuators B Chem. 2014;193:708–14.
Jia J, Xu F, Long Z, Hou X, Spepaniak MJ. Metal–organic framework MIL-53(Fe) for highly selective and ultrasensitive direct sensing of MeHg. Chem Commun. 2013;49:4670–2.
Haque E, Khan NA, Park JH, Jhung SH. Synthesis of a metal–organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: a kinetic study. Chem Eur J. 2010;16:1046–52.
Lan D, Li B, Zhang Z. Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens Bioelectron. 2008;24:934–8.
Chaichi MJ, Ehsani M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol–H2O2–gold nanoparticle chemiluminescence detection system. Sensors Actuators B Chem. 2016;223:713–22.
Chen W, Hong L, Liu AL, Liu JQ, Lin XH, Xia XH. Enhanced chemiluminescence of the luminol-hydrogen peroxide system by colloidal cupric oxide nanoparticles as peroxidase mimic. Talanta. 2012;99:643–8.
Hao M, Liu N, Ma Z. A new luminol chemiluminescence sensor for glucose based on pH-dependent graphene oxide. Analyst. 2013;138:4393–7.
Zargoosh K, Chaichi MJ, Shamsipur M, Hossienkhanid S, Asghari S, Qandalee M. Highly sensitive glucose biosensor based on the effective immobilization of glucose oxidase/carbon-nanotube and gold nanoparticle in Nafion film and peroxyoxalate chemiluminescence reaction of a new fluorophore. Talanta. 2012;93:37–43.
Yu D, Wang P, Zhao Y, Fan A. Iodophenol blue-enhanced luminol chemiluminescence and its application to hydrogen peroxide and glucose detection. Talanta. 2016;146:655–61.
Díez P, Piuleac C-G, Martínez-Ruiz P, Romano S, Gamella M, Villalonga R, et al. Supramolecular immobilization of glucose oxidase on gold coated with cyclodextrin-modified cysteamine core PAMAM G-4 dendron/Pt nanoparticles for mediatorless biosensor design. Anal Bioanal Chem. 2013;405:3773–81.
Zhang H, Huang H, Lin Z, Su X. A turn-on fluorescence-sensing technique for glucose determination based on graphene oxide–DNA interaction. Anal Bioanal Chem. 2014;406:6925–32.
Hu L, Yuan Y, Zhang L, Zhao J, Majeed S, Xu G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta. 2013;762:83–6.
Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H, et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695–7.
Ai L, Zhang C, Li L, Jiang J. Iron terephthalate metal–organic framework: revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl Catal B Environ. 2014;148–149:191–200.
Acknowledgments
Financial support from the National Natural Science Foundation of China (21277111 and 21465024) and the Fundamental Research Funds for the Central Universities (XDJK 2016D019) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Published in the topical collection Highlights of Analytical Chemical Luminescence with guest editors Aldo Roda, Hua Cui, and Chao Lu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 238 kb)
Rights and permissions
About this article
Cite this article
Yi, X., Dong, W., Zhang, X. et al. MIL-53(Fe) MOF-mediated catalytic chemiluminescence for sensitive detection of glucose. Anal Bioanal Chem 408, 8805–8812 (2016). https://doi.org/10.1007/s00216-016-9681-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9681-y