Abstract
Passiflora incarnata as well as some other Passiflora species are reported to possess anxiolytic and sedative activity and to treat various CNS disorders. The medicinal use of only a few Passiflora species has been scientifically verified. There are over 400 species in the Passiflora genus worldwide, most of which have been little characterized in terms of phytochemical or pharmacological properties. Herein, large-scale multi-targeted metabolic profiling and fingerprinting techniques were utilized to help gain a broader insight into Passiflora species leaves’ chemical composition. Nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS) spectra of extracted components derived from 17 Passiflora accessions and from different geographical origins were analyzed using multivariate data analyses. A total of 78 metabolites were tentatively identified, that is, 20 C-flavonoids, 8 O-flavonoids, 21 C, O-flavonoids, 2 cyanogenic glycosides, and 23 fatty acid conjugates, of which several flavonoid conjugates are for the first time to be reported in Passiflora spp. To the best of our knowledge, this study provides the most complete map for secondary metabolite distribution within that genus. Major signals in 1H-NMR and MS spectra contributing to species discrimination were assigned to those of C-flavonoids including isovitexin-2″-O-xyloside, luteolin-C-deoxyhexoside-O-hexoside, schaftoside, isovitexin, and isoorientin. P. incarnata was found most enriched in C-flavonoids, justifying its use as an official drug within that genus. Compared to NMR, LC-MS was found more effective in sample classification based on genetic and/ or geographical origin as revealed from derived multivariate data analyses. Novel insight on metabolite candidates to mediate for Passiflora CNS sedative effects is also presented.

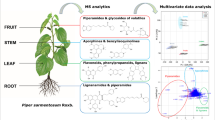
A comparative NMR and MS spectra of extracted components derived from 17 Passiflora accessions to be analyzed using multivariate data analyses for species classification.





Similar content being viewed by others
References
Simpson MG. Plant systematics. 2nd ed. Burlington, MA: Academic; 2010.
Mabberley DJ. The plant-book: a portable dictionary of the vascular plants. 2nd ed. New York: Cambridge University Press; 1997.
Brasseur T, Angenot L. The pharmacognosy of the passion flower. J Pharm Belg. 1984;39(1):15–22.
Bergner P. Passionflower. Med Herbalism. 1995;7:13–4.
Ingale AG, Hivrale AU. Pharmacological studies of Passiflora sp. and their bioactive compounds. African J Plant Sci. 2010;4:417–26.
Lakhan SE, Vieira KF. Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutr J. 2010;9(42):1–14. doi:10.1186/1475-2891-9-42.
Fiebich BL, Knoerle R, Appel K, Kammler T, Weiss G. Pharmacological studies in an herbal drug combination of St. John’s Wort (Hypericum perforatum) and passion flower (Passiflora incarnata): in vitro and in vivo evidence of synergy between Hypericum and Passiflora in antidepressant pharmacological models. Fitoterapia. 2011;82(3):474–80. doi:10.1016/j.fitote.2010.12.006.
Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21(12):841–60. doi:10.1016/j.euroneuro.2011.04.002.
Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. J Ethnopharmacol. 2004;94(1):1–23. doi:10.1016/j.jep.2004.02.023.
Wessjohann LA, Keim J, Weigel B, Dippe M. Alkylating enzymes. Curr Opin Chem Biol. 2013;17(2):229–35. doi:10.1016/j.cbpa.2013.02.016.
Aoyagi N, Kimura R, Murata T. Studies on Passiflora incarnata dry extract. I. Isolation of maltol and pharmacological action of maltol and ethyl maltol. Chem Pharm Bull. 1974;22(5):1008–13.
Lutomski J, Malek B. Pharmacochemical investigations on raw materials genus Passiflora. 3. Phytochemical investigations on raw materials of Passiflora edulis forma flavicarpa (Pharmakochemische Untersuchungen von Drogen der Gattung Passiflora. 3. Mitteilung: Phytochemische Forschung der Drogen aus Passiflora edulis forma flavicarpa). Planta Med. 1975;27(3):222–5.
Congora C, Proliac A, Raynaud J. Isolation and identification of two mono-C-glucosyl luteolins and of the di-C-substituted 6,8-diglucosyl luteolin from the leafy stalks of P. incarnanta L. Helv Chim Acta. 1986;69:251–3.
Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora caerulea. Pharmacol Biochem Behav. 1994;47(1):1–4.
Farag MA, Porzel A, Wessjohann LA. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry. 2012;76:60–72. doi:10.1016/j.phytochem.2011.12.010.
Okada T, Nakamura Y, Kanaya S, Takano A, Malla KJ, Nakane T, et al. Metabolome analysis of Ephedra plants with different contents of ephedrine alkaloids by using UPLC-Q-TOF-MS. Planta Med. 2009;75(12):1356–62. doi:10.1055/s-0029-1185577.
Farag M, Mahrous E, Lübken T, Porzel A, Wessjohann L. Classification of commercial cultivars of Humulus lupulus L. (hop) by chemometric pixel analysis of two dimensional nuclear magnetic resonance spectra. Metabolomics. 2013;10(1):21–32. doi:10.1007/s11306-013-0547-4.
Farag MA, Porzel A, Schmidt J, Wessjohann LA. Metabolite profiling and fingerprinting of commercial cultivars of L. (hop): a comparison of MS and NMR methods in metabolomics. Metabolomics. 2012;8(3):492–507. doi:10.1007/s11306-011-0335-y.
Farag MA, Wessjohann LA. Metabolome classification of commercial Hypericum perforatum (St. John’s Wort) preparations via UPLC-qTOF-MS and chemometrics. Planta Med. 2012;78(5):488–96. doi:10.1055/s-0031-1298170.
Farag MA, Porzel A, Mahrous EA, El-Massry MM, Wessjohann LA. Integrated comparative metabolite profiling via MS and NMR techniques for Senna drug quality control analysis. Anal Bioanal Chem. 2015;407(7):1937–49. doi:10.1007/s00216-014-8432-1.
Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–87. doi:10.1021/ac051437y.
Li H, Zhou P, Yang Q, Shen Y, Deng J, Li L, et al. Comparative studies on anxiolytic activities and flavonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’. J Ethnopharmacol. 2011;133(3):1085–90. doi:10.1016/j.jep.2010.11.039.
McCormick S, Mabry TJ. Flavonoids of Passiflora pavonis. J Nat Prod. 1981;44(5):623–4. doi:10.1021/np50017a025.
Geiger H, Markham KR. The C-glycosylflavone pattern of Passiflora incarnata. Z Naturforsch C. 1986;41:949–50.
Patel SS, Soni H, Mishra K, Singhai AK. Recent updates on the genus Passiflora: a review. Int J Res Phytochem Pharmacol. 2011;1(1):1–16.
Simirgiotis MJ, Schmeda-Hirschmann G, Borquez J, Kennelly EJ. The Passiflora tripartita (banana passion) fruit: a source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Molecules. 2013;18(2):1672–92. doi:10.3390/molecules18021672.
Ulubelen A, Mabry TJ. C-Glycosylflavonoids of Passiflora serratifolia. J Nat Prod. 1980;43(1):162–3. doi:10.1021/np50007a017.
Escobar LK, Liu Y-L, Mabry TJ. C-glycosylflavonoids from Passiflora coactilis. Phytochemistry. 1983;22(3):796–7. doi:10.1016/S0031-9422(00)86995-2.
McCormick S, Mabry TJ. O- and C-glycosylflavones from Passiflora biflora. Phytochemistry. 1983;22(3):798–9. 10.1016/S0031-9422(00)86996-4.
Sakalem ME, Negri G, Tabach R. Chemical composition of hydroethanolic extracts from five species of the Passiflora genus. Revista Brasileira De Farmacognosia (Braz J Pharmacogn). 2012;22(6):1219–32. doi:10.1590/s0102-695x2012005000108.
Ferreres F, Sousa C, Valentao P, Andrade PB, Seabra RM, Gil-Izquierdo A. New C-deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agric Food Chem. 2007;55(25):10187–93. doi:10.1021/jf072119y.
Zhao J, Pawar RS, Ali Z, Khan IA. Phytochemical investigation of Turnera diffusa. J Nat Prod. 2007;70(2):289–92. doi:10.1021/np060253r.
Mareck U, Herrmann K, Galensa R, Wray V. The 6-C-chinovoside and 6-C-fucoside of luteolin from Passiflora edulis. Phytochemistry. 1991;30(10):3486–7. doi:10.1016/0031-9422(91)83241-c.
Avula B, Wang YH, Rumalla CS, Smillie TJ, Khan IA. Simultaneous determination of alkaloids and flavonoids from aerial parts of Passiflora species and dietary supplements using UPLC-UV-MS and HPTLC. Nat Prod Commun. 2012;7(9):1177–80.
de Rijke E, Out P, Niessen WMA, Ariese F, Gooijer C, Brinkman UAT. Analytical separation and detection methods for flavonoids. J Chromatogr A. 2006;1112(1–2):31–63. doi:10.1016/j.chroma.2006.01.019.
Simirgiotis MJ, Cuevas H, Tapia W, Borquez J. Edible Passiflora (banana passion) fruits: a source of bioactive C-glycoside flavonoids obtained by HSCCC and HPLC-DAD-ESI/MS/MS. Planta Med. 2012;78(11):1242–3.
Davis BD, Brodbelt JS. Determination of the glycosylation site of flavonoid monoglucosides by metal complexation and tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15(9):1287–99. doi:10.1016/j.jasms.2004.06.003.
Figueirinha A, Paranhos A, Pérez-Alonso JJ, Santos-Buelga C, Batista MT. Cymbopogon citratus leaves: characterization of flavonoids by HPLC-PDA-ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008;110(3):718–28. doi:10.1016/j.foodchem.2008.02.045.
Ferreres F, Gil-Izquierdo A, Andrade PB, Valentao P, Tomas-Barberan FA. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1161(1–2):214–23. doi:10.1016/j.chroma.2007.05.103.
Otify A, George C, Elsayed A, Farag MA. Mechanistic evidence of Passiflora edulis (Passifloraceae) anxiolytic activity in relation to its metabolite fingerprint as revealed via LC-MS and chemometrics. Food Funct. 2015;6(12):3807–17. doi:10.1039/C5FO00875A.
Abad-Garcia B, Garmon-Lobato S, Berrueta LA, Gallo B, Vicente F. New features on the fragmentation and differentiation of C-glycosidic flavone isomers by positive electrospray ionization and triple quadrupole mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(12):1834–42. doi:10.1002/rcm.3560.
Chassagne D, Crouzet J. A cyanogenic glycoside from Passiflora edulis fruits. Phytochemistry. 1998;49(3):757–9. doi:10.1016/s0031-9422(98)00130-7.
Li SS, Jiang ZM, Thamm L, Zhou GT. 10-Hydroxy-2-decenoic acid as an antimicrobial agent in draft keg-conditioned wheat beer. J Am Soc Mass Brew Chem. 2010;68:114–8.
Martin-Arjol I, Bassas-Galia M, Bermudo E, Garcia F, Manresa A. Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. Chem Phys Lipids. 2010;163(4–5):341–6. doi:10.1016/j.chemphyslip.2010.02.003.
Waluk DP, Battistini MR, Dempsey DR, Farrell EK, Jeffries KA, Mitchell P, Hernandez LW, McBride JC, Merkler DJ, Hunt MC (2014) Omega-3 fatty acids in brain and neurological health: chapter 9—mammalian fatty acid amides of the brain and CNS. Academic Press, Boston. doi:http://dx.doi.org/10.1016/B978-0-12-410527-0.00009-0
Wei XY, Yang JY, Dong YX, Wu CF. Anxiolytic-like effects of oleamide in group-housed and socially isolated mice. Prog Neuropsychopharmacol. 2007;31(6):1189–95. doi:10.1016/j.pnpbp.2007.04.008.
Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal. 2013;24(1):1–24. doi:10.1002/pca.2378.
Kuriyama K, Sze PY. Blood–brain barrier to H3-γ-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology. 1971;10(1):103–8. doi:10.1016/0028-3908(71)90013-X.
Tai Z, Cai L, Dai L, Dong L, Wang M, Yang Y, et al. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011;126(4):1648–54. doi:10.1016/j.foodchem.2010.12.048.
van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. doi:10.1186/1471-2164-7-142.
Farag MA, Deavours BE, de Fatima A, Naoumkina M, Dixon RA, Sumner LW. Integrated metabolite and transcript profiling identify a biosynthetic mechanism for hispidol in Medicago truncatula cell cultures. Plant Physiol. 2009;151(3):1096–113. doi:10.1104/pp.109.141481.
Acknowledgments
This work was supported by the Alexander von Humboldt-Foundation, Germany to Dr. Mohamed Farag and Prof. Dr. Ludger Wessjohann. Dr. Mohamed Ali Farag acknowledges the funding received by Science and Technology Development fund STDF, Egypt (grant number 12594). Prof. Dr. Ludger Wessjohann and Dr. Andrea Porzel acknowledge partial support from Leibniz-Wettbewerb SAW-2015-IPB-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 840 kb)
Rights and permissions
About this article
Cite this article
Farag, M.A., Otify, A., Porzel, A. et al. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal Bioanal Chem 408, 3125–3143 (2016). https://doi.org/10.1007/s00216-016-9376-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9376-4