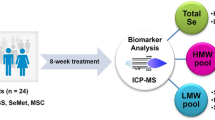
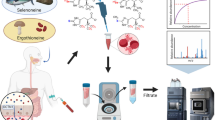
Abstract
The paper presents an analytical method for quantification of low molecular weight (LMW) selenium compounds in human plasma based on liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP-MS) and post column isotope dilution-based quantification. Prior to analysis, samples were ultrafiltrated using a cut-off value of 3000 Da. The method was validated in aqueous solution as well as plasma using standards of selenomethionine (SeMet), Se-methylselenocysteine (MeSeCys), selenite, and the selenosugar Se-methylseleno-N-acetylgalactosamine (SeGal) for linearity, precision, recoveries, and limits of detection and quantitation with satisfactory results. The method was applied for analysis of a set of plasma samples from cancer patients receiving selenite treatment in a clinical trial. Three LMW selenium compounds were observed. The main compounds, SeGal and selenite were tentatively identified by retention time matching with standards in different chromatographic systems, while the third minor compound was not identified. The identity of the selenosugar was verified by ESI-MS-MS product ion scanning, while selenite was identified indirectly as the glutathione (GSH) reaction product, GS-Se-SG.

A method for analysing plasma samples based on spin filtering and LC-ICP-MS analysis with isotope dilution quantification was developed and applied to samples from patients receiving selenite in pharmacological doses in a clinical trial






Similar content being viewed by others
References
Kryukov G, Kryukov V, Gladyshev V. J Biol Chem. 1999;274:33888–97.
Ganther HE. Carcinogenesis. 1999;20:1657–66.
Ganther HE. J Am Coll Tocicol. 1986;5:1–5.
Weekley CM, Harris HH. Chem Soc Rev. 2013;42:8870–94.
Suzuki KT. J Health Sci. 2005;51:107–14.
Gammelgaard B, Jackson MI, Gabel-Jensen C. Anal Bioanal Chem. 2011;399:1743–63.
Rayman MP. Proc Nutrition Soc. 2005;64:527–42.
Björnstedt M, Fernandes A. EPMA J. 2010;1:389–95.
Nilsonne G, Sun X, Nyström C, Rundlöf AK, Potamitou Fernandes A, Björnstedt M, et al. Free Rad Biol Med. 2006;41:874–85.
Menter DG, Sabichi AL, Lippman SM. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–82.
Husbeck B, Nonn L, Peehl DM, Knox SJ. Prostate. 2006;66:218–25.
Hanahan D, Weinberg R. Cell. 2011;144:646–74.
Brodin O, Eksborg S, Wallenberg M, Asker-Hagelberg C, Larsen EH, Molkert D, et al. Nutrient. 2015;7:4978–94.
Randomised trial to identify the safest and most effective selenium compound for cancer patients. Phase Ib randomised double-blind dose-escalation trial to identify the safest most effective selenium compound for use in patients with either chronic lymphocytic leukaemia or metastatic prostate cancer . https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000118707. 2015.
Lippman S, Klein E, Goodman P, Lucia M, Thompson I, Ford L, et al. JAMA. 2009;301:39–51.
Clark LC, Combs GF, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. JAMA. 1996;276:1957–63.
Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, et al. Am J Clin Nutr. 2010;91:923–31.
Ogra Y, Anan Y. J Anal At Spectr. 2009;24:1477–88.
Dumont E, Vanhaecke F, Cornelis R. Anal Bioanal Chem. 2006;385:1304–23.
Pedrero Z, Madrid Y. Anal Chim Acta. 2009;634:135–52.
Gammelgaard B, Gabel-Jensen C, Stürup S, Hansen HR. Anal Bioanal Chem. 2008;390:1691–706.
Lu Y, Rumpler A, Francesconi KA, Pergantis SA. Anal Chim Acta. 2012;731:49–59.
Kokarnig S, Tsirigotaki A, Wiesenhofer T, Lackner V, Francesconi KA, Pergantis SA, et al. J Trace Elem Med Biol. 2015;29:83–90.
Gonzalez-Iglesias H, Fernandez-Sanchez ML, Lu Y, Fernandez Menendez S, Pergantis SA, Sanz-Medel A. J Anal At Spectr. 2015;30:267–76.
Rodriguez-Gonzalez P, Marchante-Gayon JM, Alonso JIG, Sanz-Medel A. Spectrochim Acta Atom Spectros. 2005;60:151–207.
Berglund M, Frech W, Baxter DC. Spectrochim Acta. 1991;46B:1767–77.
Sariego Muniz C, Marchante Gayon JM, Garcia Alonso JI, Sanz-Medel A. J Anal At Spectr. 2001;16:587–92.
Foster SJ, Ganther HE. Anal Biochem. 1984;137:205–9.
Suzuki T, Shiobara Y, Itoh M, Ohmichi M. Analyst. 1998;123:63–7.
Shiobara Y, Ogra Y, Suzuki KT. Analyst. 1999;124:1237–41.
Greening DW, Simpson RJ. J Proteomics. 2010;73:637–48.
Gammelgaard B, Bendahl L, Jacobsen NW, Stürup S. J Anal At Spectr. 2005;9:889–93.
Ogra Y, Ishiwata K, Takayama H, Aimi N, Suzuki KT. J Chromatogr B. 2002;767:301–12.
Gammelgaard B, Bendahl L. J Anal At Spectr. 2004;19:135–42.
Jitaru P, Goenaga-Infante H, Vaslin-Reimann S, Fisicaro P. Anal Chim Acta. 2010;657:100–7.
Palacios O, Ruiz Encinar J, Schaumlöffel D, Lobinski R. Anal Bioanal Chem. 2006;384:1276–83.
Palacios O, Lobinski R. Talanta. 2007;71:1813–6.
Ganther HE. Biochemistry. 1968;7:2898–905.
Arteel GE, Sies H. Environ Toxicol Pharmacol. 2001;10:153–8.
Dumont E, Ogra Y, Suzuki KT, Vanhaecke F, Cornelis R. Anal Chim Acta. 2006;555:25–33.
Acknowledgments
The authors wish to thank Erik Huusfeldt Larsen for the donation of the enriched 77Se solution and laboratory technician Camilla Jensen for skillful technical assistance.
The SECAR trial was supported by grants from Cancerfonden and Jochnick Foundation, Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The SECAR-trial [14] was approved by the Swedish Medical Products Agency and registered in the EU Clinical Trial Register (Eudra CT Number: 2006-004076-13) and by the Ethical Committee of Stockholm (2006/429-31/3).
Informed consent was given by the healthy volunteer donating the plasma sample for validation.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Flouda, K., Dersch, J.M., Gabel-Jensen, C. et al. Quantification of low molecular weight selenium metabolites in human plasma after treatment with selenite in pharmacological doses by LC-ICP-MS. Anal Bioanal Chem 408, 2293–2301 (2016). https://doi.org/10.1007/s00216-016-9325-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9325-2