Abstract
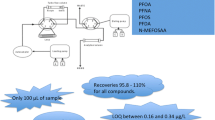
Two quantitative methods using high-performance liquid chromatography (HPLC) combined with triple quadrupole tandem mass spectrometry (MS/MS) were developed to determine perfluoroalkyl and polyfluoroalkyl substances (PFASs) in aqueous samples. The first HPLC–MS/MS method was applied to 47 PFASs of 12 different substance classes with acidic characteristics such as perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulfonic acids (PFSAs), as well as precursor substances and biotransformation intermediates (e.g., unsaturated fluorotelomer carboxylic acids). In addition, 25 13C-, 18O-, and 2H-labeled PFASs were used as internal standards in this method. The second HPLC–MS/MS method was applied to fluorotelomer alcohols (FTOHs) and perfluorooctane sulfonamidoethanols as these compounds have physicochemical properties different from those of the previous ones. Accuracy between 82% and 110% and a standard deviation in the range from 2% to 22% depending on the substances were determined during the evaluation of repeatability and precision. The method quantification limit after solid-phase extraction ranged from 0.3 to 199 ng/L depending on the analyte and matrix. The HPLC–MS/MS methods developed were suitable for the determination of PFASs in aqueous samples (e.g., wastewater treatment plant effluents or influents after solid-phase extraction). These methods will be helpful in monitoring campaigns to evaluate the relevance of precursor substances as indirect sources of perfluorinated substances in the environment. In one exemplary application in an industrial wastewater treatment plant, FTOHs were found to be the major substance class in the influent; in particular, 6:2-FTOH was the predominant compound in the industrial samples and accounted for 74% of the total PFAS concentration. The increase in the concentration of the transformation products of FTOHs in the corresponding effluent, such as fluorotelomer carboxylic acids, unsaturated fluorotelomer carboxylic acids, n:3 polyfluorinated saturated carboxylic acids (n indicates the number of nonfluorinated carbon atoms), and PFCAs, indicated biotransformation of FTOHs or their derivatives during wastewater treatment. However, only 33 mol% of the total amount of PFASs present in the influent was quantified in the corresponding effluent.

Method development of an HPLC-MS/MS multi-method for the determination of PFASs in aqueos samples



Similar content being viewed by others
References
Knepper TP, Lange FT, editors. Polyfluorinated Chemicals and transformation products. The handbook of environmental chemistry, vol. 17. Heidelberg: Springer; 2012.
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–41. doi:10.1002/ieam.258.
Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharm. 2004;198(2):231–41. doi:10.1016/j.taap.2003.11.031.
Grandjean P, Budtz-Jorgensen E. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ Health. 2013;12(1):35.
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. doi:10.1093/toxsci/kfm128.
DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40(2):300–11. doi:10.1177/0192623311428473.
Europe Commission. Commission Regulation (EU) No 757/2010 of 24 August 2010 amending Regulation (EC) No 850/2004 of the European Parliament and of the Council on persistent organic pollutants as regards Annexes I and III. 2010. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:223:0029:0036:en:PDF. Accessed 28 Jan 2015.
European Chemicals Agency. inclusion of substances of very high concern in the candidate list (decision of the European Chemicals Agency). 2013. http://echa.europa.eu/documents/10162/b54352de-0f2f-454c-bc83-04f191c560b7. Accessed 25 Aug 2015.
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40(1):32–44. doi:10.1021/Es0512475.
Frömel T, Knepper TP. Biodegradation of fluorinated alkyl substances. Rev Environ Contam Toxicol. 2010;208:161–7.
Chen H, Zhang C, Han J, Yu Y, Zhang P. PFOS and PFOA in influents, effluents, and biosolids of Chinese wastewater treatment plants and effluent-receiving marine environments. Environ Pollut. 2012;170:26–31. doi:10.1016/j.envpol.2012.06.016.
Sinclair E, Kannan K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ Sci Technol. 2006;40(5):1408–14. doi:10.1021/Es051798v.
Pan YY, Shi YL, Wang JM, Cai YQ. Evaluation of perfluorinated compounds in seven wastewater treatment plants in Beijing urban areas. Sci China Chem. 2011;54(3):552–8. doi:10.1007/s11426-010-4093-x.
Kunacheva C, Tanaka S, Fujii S, Boontanon SK, Musirat C, Wongwattana T, et al. Mass flows of perfluorinated compounds (PFCs) in central wastewater treatment plants of industrial zones in Thailand. Chemosphere. 2011;83(6):737–44. doi:10.1016/j.chemosphere.2011.02.059.
Wang N, Szostek B, Buck RC, Folsom PW, Sulecki LM, Gannon JT. 8-2 Fluorotelomer alcohol aerobic soil biodegradation: pathways, metabolites, and metabolite yields. Chemosphere. 2009;75(8):1089–96. doi:10.1016/j.chemosphere.2009.01.033.
Wang N, Szostek B, Folsom PW, Sulecki LM, Capka V, Buck RC, et al. Aerobic biotransformation of C-14-labeled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ Sci Technol. 2005;39(2):531–8. doi:10.1021/Es049466y.
Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol. 2004;38(10):2857–64. doi:10.1021/Es0350177.
Andersen MPS, Nielsen OJ, Hurley MD, Ball JC, Wallington TJ, Ellis DA, et al. Atmospheric chemistry of 4:2 fluorotelomer alcohol (n-C4F9CH2CH2OH): products and mechanism of Cl atom initiated oxidation in the presence of NOx. J Phys Chem A. 2005;109(9):1849–56. doi:10.1021/Jp045672g.
Wallington TJ, Hurley MD, Xia J, Wuebbles DJ, Sillman S, Ito A, et al. Formation of C7F15COOH (PFOA) and other perfluorocarboxylic acids during the atmospheric oxidation of 8:2 fluorotelomer alcohol. Environ Sci Technol. 2006;40(3):924–30. doi:10.1021/Es051858x.
Frömel T. Biotransformation, trace analysis and effects of perfluoroalkyl and polyflouroalkyl substances. Berlin: Technische Universität Berlin; 2012.
Frömel T, Gremmel C, Dimzon IK, Weil H, de Voogt P, Knepper PT. Investigations on the presence and behavior of precursors to perfluoroalkyl substances in the environment as a preparation of regulatory measures. Dessau-Roßlau: Environmental Research of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety; 2016.
Boone JS, Guan B, Vigo C, Boone T, Byrne C, Ferrario J. A method for the analysis of perfluorinated compounds in environmental and drinking waters and the determination of their lowest concentration minimal reporting levels. J Chromatogr A. 2014;1345:68–77.
Gremmel C, Frömel T, Knepper TP. Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere. 2016;160:173–80. doi:10.1016/j.chemosphere.2016.06.043.
Möller A, Ahrens L, Surm R, Westerveld J, van der Wielen F, Ebinghaus R, et al. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ Pollut. 2010;158(10):3243–50. doi:10.1016/j.envpol.2010.07.019.
Kirchgeorg T, Weinberg I, Dreyer A, Ebinghaus R. Perfluorinated compounds in marine surface waters: data from the Baltic Sea and methodological challenges for future studies. Environ Chem. 2010;7:429–34.
Dreyer A, Thuens S, Kirchgeorg T, Radke M. Ombrotrophic Peat Bogs are not suited as natural archives to investigate the historical atmospheric deposition of perfluoroalkyl substances. Environ Sci Technol. 2012;46(14):7512–9. doi:10.1021/es204175y.
Ullah S, Alsberg T, Berger U. Simultaneous determination of perfluoroalkyl phosphonates, carboxylates, and sulfonates in drinking water. J Chromatogr A. 2011;1218(37):6388–95. doi:10.1016/j.chroma.2011.07.005.
Frömel T, Knepper TP. Mass spectrometry as an indispensable tool for studies of biodegradation of surfactants. Trends Anal Chem. 2008;27(11):1091–106. doi:10.1016/j.trac.2008.09.012.
Ahrens L, Felizeter S, Sturm R, Xie Z, Ebinghaus R. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Mar Pollut Bull. 2009;58:1326–33.
Kim S-K, Im J-K, Kang Y-M, Jung S-Y, Kho YL, Zoh K-D. Wastewater treatment plants (WWTPs)-derived national discharge loads of perfluorinated compounds (PFCs). J Hazard Mater. 2012;201–202:82–91.
Vierke L, Ahrens L, Shoeib M, Reiner EJ, Guo R, Palm W-U, et al. Air concentrations and particle–gas partitioning of polyfluoroalkyl compounds at a wastewater treatment plant. Environ Chem. 2011;8:363–71. doi:10.1071/EN10133.
Ahrens L, Shoeib M, Harner T, Lee SC, Guo R, Reiner EJ. Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere. Environ Sci Technol. 2011;45(19):8098–105. doi:10.1021/es1036173.
Acknowledgements
We thank the German Environment Agency (Dessau, Germany) for financial support of the research project “Investigations on the presence and behavior of precursors to perfluoroalkyl substances in the environment as a preparation of regulatory measures” (FKZ 3712 65 415/01); PFC_EXPO. We thank Lena Vierke and Annegret Biegel-Engler of the German Environment Agency for helpful discussions. We also like thank the operators of the wastewater treatment plant for their support during the planning and realization of the sampling campaigns. We are very thankful to Robert Buck and Ning Wang from DuPont for providing the reference material for n:3-acids as well as the isotopically labeled standards used in the HPLC–MS/MS-n method.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 365 kb)
Rights and permissions
About this article
Cite this article
Gremmel, C., Frömel, T. & Knepper, T.P. HPLC–MS/MS methods for the determination of 52 perfluoroalkyl and polyfluoroalkyl substances in aqueous samples. Anal Bioanal Chem 409, 1643–1655 (2017). https://doi.org/10.1007/s00216-016-0110-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0110-z