Abstract
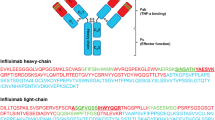
Infliximab (IFX) is a chimeric monoclonal antibody targeting tumor necrosis factor-alpha. It is currently approved for the treatment of certain rheumatic diseases or inflammatory bowel diseases. Clinical studies have suggested that monitoring IFX concentrations could improve treatment response. However, in most studies, IFX was quantified using ELISA assays, the resulting discrepancies of which raised concerns about their reliability. Here, we describe the development and validation of a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for IFX quantification in human plasma. Full-length stable-isotope-labeled antibody (SIL-IFX) was added to plasma samples as internal standard. Samples were then prepared using Mass Spectrometry Immuno Assay (MSIA™) followed by trypsin digestion and submitted to multiple reaction monitoring (MRM) for quantification of IFX. The chromatographic run lasted 13 min. The range of quantification was 1 to 26 mg/L. For two internal quality controls spiked with 6 and 12 mg/L of IFX, the method was reproducible (coefficients of variation (CV%): 12.7 and 2.1), repeatable (intra-day CV%: 5.5 and 5.0), and accurate (inter-day and intra-day deviations from nominal values: +6.4 to +3.7 % and 5.5 to 9.2 %, respectively). There was no cross - contamination effect. Samples from 45 patients treated with IFX were retrospectively analyzed by LC-MS/MS and results were compared to those obtained with an in-house ELISA assay and the commercial Lisa Tracker® method. Good agreement was found between LC-MS/MS and in-house ELISA (mean underestimation of 13 % for in-house ELISA), but a significant bias was found with commercial ELISA (mean underestimation of 136 % for commercial ELISA). This method will make it possible to standardize IFX quantification between laboratories.

Interassay comparison of the three methods: LC-MS/MS vs inhouse ELISA assay or vs Lisa Tracker® ELISA assays, Passing & Bablok (a) and Bland & Altman (b) for the comparison of LC-MS/MS vs in-house ELISA assay; Passing & Bablok (c); Bland & Altman (d) for the comparison LC-MS/MS vs Lisa Tracker® ELISA assay






Similar content being viewed by others
References
Afif W, Loftus EV, Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi:10.1038/ajg.2010.9.
Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–307.e5. doi:10.1053/j.gastro.2014.08.035.
Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–307.e3. doi:10.1053/j.gastro.2015.02.031.
Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi:10.1136/gut.2009.183095.
van Bezooijen JS, Koch BCP, van Doorn MBA, Prens EP, van Gelder T, Schreurs MWJ. Comparison of three assays to quantify infliximab, adalimumab, and etanercept serum concentrations. Ther Drug Monit. 2016;38:432–8. doi:10.1097/FTD.0000000000000310.
Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118–24.e3. doi:10.1016/j.cgh.2014.12.026.
Steenholdt C, Ainsworth MA, Tovey M, Klausen TW, Thomsen OØ, Brynskov J, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohnʼs disease. Ther Drug Monit. 2013;35:530–8. doi:10.1097/FTD.0b013e31828d23c3.
Lee MWM, Connor S, Ng W, Toong CM-L. Comparison of infliximab drug measurement across three commercially available ELISA kits. Pathology. 2016;48:608–12. doi:10.1016/j.pathol.2016.07.001.
Wu AHB, French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin Chim Acta. 2013;420:4–10. doi:10.1016/j.cca.2012.10.026.
Grebe SKG, Singh RJ. LC-MS/MS in the clinical laboratory—where to from here? Clin Biochem Rev. 2011;32:5–31.
Rauh M. LC–MS/MS for protein and peptide quantification in clinical chemistry. J Chromatogr B. 2012;883–884:59–67. doi:10.1016/j.jchromb.2011.09.030.
Xu K, Liu L, Maia M, Li J, Lowe J, Song A, et al. A multiplexed hybrid LC–MS/MS pharmacokinetic assay to measure two co-administered monoclonal antibodies in a clinical study. Bioanalysis. 2014;6:1781–94. doi:10.4155/bio.14.142.
Lebert D, Picard G, Beau-Larvor C, Troncy L, Lacheny C, Maynadier B, et al. Absolute and multiplex quantification of antibodies in serum using PSAQ™ standards and LC-MS/MS. Bioanalysis. 2015;7:1237–51. doi:10.4155/bio.15.56.
Ramagiri S, Moore I. Hybridizing LBA with LC–MS/MS: the new norm for biologics quantification. Bioanalysis. 2016;8:483–6. doi:10.4155/bio.16.9.
Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F. Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography−tandem mass spectrometry. Anal Chem. 2008;80:1290–6. doi:10.1021/ac702115b.
Willrich MAV, Murray DL, Barnidge DR, Ladwig PM, Snyder MR. Quantitation of infliximab using clonotypic peptides and selective reaction monitoring by LC–MS/MS. Int Immunopharmacol. 2015;28:513–20. doi:10.1016/j.intimp.2015.07.007.
Grebe SKG, Singh RJ. Clinical peptide and protein quantification by mass spectrometry (MS). TrAC Trends Anal Chem. 2016;1–13. doi:10.1016/j.trac.2016.01.026.
Inc. TFS. Thermo Scientific MSIA Streptavidin D.A.R.T.’S. 2013. https://tools.thermofisher.com/content/sfs/manuals/D21140~.pdf.
Qian W-J, Jacobs JM, Liu T, Camp DG, Smith RD. Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol Cell Proteomics. 2006;5:1727–44. doi:10.1074/mcp.M600162-MCP200.
U.S. Department of Health and Human Services Food and Drug Administration. Guidance for industry bioanalytical method validation guidance for industry bioanalytical method validation. 2001. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–4.
Ternant D, Mulleman D, Degenne D, Willot S, Guillaumin J-M, Watier H, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit. 2006;28:169–74. doi:10.1097/01.ftd.0000189901.08684.4b.
Theradiag Lisa-Tracker—Monitoring of patients under biotherapies. http://www.theradiag.com/en/files/2016/04/6-pages-LISA-TRACKERv01-2016-UK-planches.pdf.
Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7.
Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9.e3. doi:10.1053/j.gastro.2015.02.031.
Kobayashi T, Suzuki Y, Motoya S, Hirai F, Ogata H, Ito H, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51:241–51. doi:10.1007/s00535-015-1102-z.
van den Bemt BJF, den Broeder AA, Wolbink G-J, van den Maas A, Hekster YA, van Riel PLCM, et al. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol. 2013;76:939–45. doi:10.1111/bcp.12142.
Schmitz EMH, van de Kerkhof D, Hamann D, van Dongen JLJ, Kuijper PHM, Brunsveld L, et al. Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin Chem Lab Med. 2016. doi:10.1515/cclm-2015-0987.
Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe. MAbs. 2013;5:621–3. doi:10.4161/mabs.25864.
Beck A, Debaene F, Diemer H, Wagner-Rousset E, Colas O, Van Dorsselaer A, et al. Cutting-edge mass spectrometry characterization of originator, biosimilar and biobetter antibodies. J Mass Spectrom. 2015;50:285–97. doi:10.1002/jms.3554.
Papamichael K, Van Stappen T, Vande Casteele N, Gils A, Billiet T, Tops S, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–9. doi:10.1016/j.cgh.2015.11.014.
Vande Casteele N, Buurman DJ, Sturkenboom MGG, Kleibeuker JH, Vermeire S, Rispens T, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–71. doi:10.1111/apt.12030.
Guiotto C, Daperno M, Frigerio F, Vizzini M, Cerruti R, Ercole E, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis. 2016;48:138–43. doi:10.1016/j.dld.2015.10.023.
Bults P, van de Merbel NC, Bischoff R. Quantification of biopharmaceuticals and biomarkers in complex biological matrices: a comparison of liquid chromatography coupled to tandem mass spectrometry and ligand binding assays. Expert Rev Proteomics. 2015;12:355–74. doi:10.1586/14789450.2015.1050384.
Acknowledgments
We thank Ravindra Chaudhari from Thermo-Fisher for advice and technical assistance with the MSIA procedure. We are also indebted to Karine Scalabrino for excellent relevant technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the Grenoble University Hospital ethics committee IRB6705 and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 53 kb)
Rights and permissions
About this article
Cite this article
Jourdil, JF., Lebert, D., Gautier-Veyret, E. et al. Infliximab quantitation in human plasma by liquid chromatography-tandem mass spectrometry: towards a standardization of the methods?. Anal Bioanal Chem 409, 1195–1205 (2017). https://doi.org/10.1007/s00216-016-0045-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0045-4