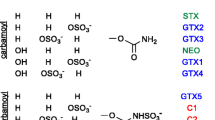
Abstract
The analysis of paralytic shellfish toxins (PSTs) by liquid chromatography-mass spectrometry remains a challenge because of their high polarity, large number of analogues and the complex matrix in which they occur. Here we investigate the potential utility of high-field asymmetric waveform ion mobility spectrometry (FAIMS) as a gas-phase ion separation tool for analysis of PSTs by mass spectrometry. We investigate the separation of PSTs using FAIMS with two divergent goals: using FAIMS as a primary separation tool for rapid screening by electrospray ionization (ESI)-FAIMS-MS or combined with LC in a multidimensional LC-ESI-FAIMS-MS separation. First, a survey of the parameters that affect the sensitivity and selectivity of PST analysis by FAIMS was carried out using ESI-FAIMS-MS. In particular, the use of acetonitrile as a gas additive in the carrier gas flow offered good separation of all PST epimeric pairs. A second set of FAIMS conditions was also identified, which focussed PSTs to a relatively narrow CV range allowing development of an LC-ESI-FAIMS-MS method for analysis of PST toxins in complex mussel tissue extracts. The quantitative capabilities of this method were evaluated by analysing a PST containing mussel tissue matrix material. Results compared favourably with analysis by an established LC–post-column oxidation–fluorescence method with recoveries ranging from 70 to 106 %, although sensitivity was somewhat reduced. The current work represents the first successful separation of PST isomers using ion mobility and shows the promise of FAIMS as a tool for analysis of algal biotoxins in complex samples and outlines some critical requirements for its future improvement.







Similar content being viewed by others
References
Vale P (2014) Saxitoxin and analogs: ecobiology, origin, chemistry, and detection. In: Botana LM (ed) Seafood and freshwater toxins: pharmacology physiology and detection, 3rd edn. CRC Press, Boca Raton
Quilliam MA (2003) The role of chromatography in the hunt for red tide toxins. J Chromatogr A 1000:527–548
MBA, AOAC 959.08 Official Methods of Analysis (2000) 17th edn. AOAC INTERNATIONAL, Gaithersburg, MD. Method 959.08
Hess P, Grune B, Anderson DA, Aune T, Botana LM, Caricato P, van Egmond HP, Halder M, Hall S, Lawrence JF, Moffat C, Poletti R, Richmond J, Rossini GP, Seamer C, Vilageliu JS (2006) Three Rs approaches in marine biotoxin testing. Altern Lab Anim 34:193–224
Anonymous (2011) Commission Regulation (EU) No 15/2011 of 10 January 2011 amending Regulation (EC) No 2074/2005 as regards recognised testing methods for detecting marine biotoxins in live bivalve molluscs. Official Journal of the European Union L 006 of 1 January 2011: 3–6
Anonymous (2005) AOAC official method 2005.06 quantitative determination of paralytic shellfish poisoning toxins in shellfish using pre-chromatographic oxidation and liquid chromatography with fluorescence detection. AOAC International, Gaithersburg
Rourke WA, Murphy CJ, Pitcher G, van de Riet JM, Burns BG, Thomas KM, Quilliam MA (2008) Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. J AOAC Int 91:589–597
Van de Riet JM, Gibbs RS, Chou FW, Muggah FW, Rourke WA, Burns G, Thomas G, Quilliam MA (2009) Liquid chromatographic post-column oxidation method for analysis of paralytic shellfish toxins in mussels, clams, scallops and oysters: single-laboratory validation. J AOAC Int 92:1690–1704
Brana-Magdalena A, Leao-Martins JM, Glauner T, Gago-Martinez A (2014) Intralaboratory validation of a fast and sensitive UHPLC/MS/MS method with fast polarity switching for the analysis of lipophilic shellfish toxins. J AOAC Int 97:285–292
McNabb P, Selwood AI, Holland PT, Aasen J, Aune T, Eaglesham G, Hess P, Igarishi M, Quilliam M, Slattery D, Van de Riet J, Van Egmond H, Van den Top H, Yasumoto T (2005) Multiresidue method for determination of algal toxins in shellfish: single-laboratory validation and interlaboratory study. J AOAC Int 88(3):761–772
van den Top HJ, Gerssen A, McCarron P, van Egmond HP (2011) Quantitative determination of marine lipophilic toxins in mussels, oysters and cockles using liquid chromatography-mass spectrometry: inter-laboratory validation study. Food Addit Contam Part A 28(12):1745–1757
Dell’Aversano C, Hess P, Quilliam MA (2005) Hydrophilic interaction liquid chromatography-mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J Chromatogr A 1081:190–201
Zhou L, Yin Y, Fu W, Qui B, Lin, Yang Y, Zheng L, Li J, Chen G (2013) Determination of paralytic shellfish poisoning toxins by HILIC–MS/MS coupled with dispersive solid phase extraction. Food Chem 137:115–121
Blay P, Hui JPM, Chang J, Melanson JE (2011) Screening for multiple classes of marine biotoxins by liquid chromatography–high-resolution mass spectrometry. Anal Bioanal Chem 400:577–585
Watanabe R, Matsushima R, Harada T, Oikawa H, Murata M, Suzuki T (2013) Quantitative determination of paralytic sellfish toxins in cultured toxic algae by LC-MS/MS. Food Addit Contam Part A 8:1351–1357
Purves R, Guevremont R, Day S, Pipich CW, Matyjaszczyk S (1998) Mass spectrometric characterization of a high-field asymmetric waveform ion mobility spectrometer. Rev Sci Instrum 69:4094–4105
Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG (2010) Chemical effects in the separation process of a differential mobility/mass spectrometer system. Anal Chem 82:1867–1880
Prasad S, Belford M, Dunyach J, Purves R (2014) On an aerodynamic mechanism to enhance ion transmission and sensitivity of FAIMS for nano-electrospray ionization-mass spectrometry. J Am Soc Mass Spectrom 25:2143–2153
Shvartsburg AA, Smith RD, Wilks A, Koehl A, Ruiz-Alsonso D, Boyl B (2009) Ultrafast differential ion mobility spectrometry at extreme electric fields in multichannel microchips. Anal Chem 81:6489–6495
Purves RW, Ozog A, Ambrose SJ, Prasad S, Belford M, Dunyach J (2014) Using gas modifiers to significantly improve sensitivity and selectivity in a cylindrical FAIMS device. J Am Soc Mass Spectrom 25:1274–12844
Shvartsburg AA (2009) Differential ion mobility spectrometry. CRC Press, Boca Raton
Purves RW (2013) Enhancement of biological mass spectrometry by using separations based on changes in ion mobility (FAIMS and DMS). Anal Bioanal Chem 405:35–42
Buryakov IA, Krylov EV, Nazarov EG, Rasulev UK (1993) A new method of separation of multi-atomic ions by mobility at atmospheric pressure using a high-frequency amplitude-asymmetric strong electric field. Int J Mass Spectrom Ion Process 128:143–148
Gabryelski W, Wu F, Froese KL (2008) Comparison of high-field asymmetric waveform ion mobility spectrometry with GC methods in analysis of haloacetic acids in drinking water. Anal Chem 75:2478–2486
Purves RW, Guevremont R (1999) Electrospray ionization high-field asymmetric waveform ion mobility spectrometry-mass spectrometry. Anal Chem 71:2346–2357
Ells B, Barnett DA, Purves RW, Guevremont R (2000) Detection of nine chlorinated and brominated haloacetic acids at part-per-trillion levels using ESI-FAIMS-MS. Anal Chem 72:5455–4559
Guevremont R, Purves RW, Barnett DA, Ells B (2006) FAIMS apparatus and method using carrier gases that contain a trace amount of a dopant species. US Patent no. 7,026,612
Rorrer LCIII, Yost RA (2011) Solvent vapour effects on planar high-field asymmetric waveform ion mobility spectrometry. Int J Mass Spectrom 300:173–181
Poyer S, Loutelier-Bourhis C, Mondeguer F, Enche J, Coadou G, Bossée A, Hess P, Afonso C (2014) Characterization of paralytic shellfish poisons by HILIC-IM-MS coupling. 62nd ASMS Conference on Mass Spectrometry and Allied Topics, Baltimore, MD
Reeves K, Thomas K, Quilliam MA (2004) Mussel tissue certified reference material for paralytic shellfish poisoning toxins. Proceedings of the 5th International Conference on Molluscan Shellfish Safety. Marine Institute, pg. 116–122. http://hdl.handle.net/10793/576. Accessed 23 May 2014
Dorr FA, Kovacevic B, Maksic ZB, Pinto E, Volmer DA (2011) Intriguing differences in the gas-phase dissociation behavior of protonated and deprotonated gonyautoxin epimers. J Am Soc Mass Spectrom 22:2011–2020
Barnett DA, Ells B, Guevremont R, Purves RW, Viehland LA (2000) Evaluation of carrier gases for use in high-field asymmetric waveform ion mobility spectrometry. J Am Soc Mass Spectrom 11:1125–1133
Hall AB, Coy SL, Kafle A, Glick J, Nazarov E, Vouros P (2013) Extending the dynamic range of the ion trap by differential mobility filtration. J Am Soc Mass Spectrom 24:1428–1436
Barnett DA, Belford M, Dunyach J, Purves RE (2007) Characterization of a temperature-controlled FAIMS system. J Am Soc Mass Spectrom 18:1653–1663
Acknowledgments
The authors gratefully acknowledge the technical assistance of Krista Thomas and Margaret McCooeye as well as the support and editorial assistance of Pearse McCarron and Michael Quilliam. The authors would like to thank Thermo Fisher for the loan of the TSQ Quantum.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 156 kb)
Rights and permissions
About this article
Cite this article
Beach, D.G., Melanson, J.E. & Purves, R.W. Analysis of paralytic shellfish toxins using high-field asymmetric waveform ion mobility spectrometry with liquid chromatography-mass spectrometry. Anal Bioanal Chem 407, 2473–2484 (2015). https://doi.org/10.1007/s00216-015-8488-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8488-6