Abstract
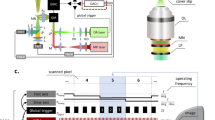
Tip-enhanced Raman scattering (TERS) was paired with real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) to characterize lipid aggregates during stimulated re-epithelialization using an in vitro wound healing model. In this study, lipid fluctuations in the plasma membrane of epidermal keratinocytes were studied at multiple time points post-wounding. TERS measurements for the first time were also combined with sample analysis after initial wounding and 24 h of wound healing. This enabled simultaneous visualization and characterization of caveolar bulb distribution during wound healing stages, providing noninvasive insight into their associated lipid structure and coating protein, caveolin, in the nanometer range. The combination of Raman spectroscopy and scanning probe microscopy in TERS gives access to topographic and chemical structure information in a single experiment. It is the intrinsic specificity and sensitivity of TERS that enable this discrete detection of cell surface components on the nanometer scale. In contrast with competing biochemical methods, the applied technique does not interfere with the cellular composition, enabling lipid structure analysis without digestion or detergents, and displayed great potential for future biological in vivo studies.

tip_enhanced Raman spectroscopy of cell caveolar bulbs formed on a wound healing model






Similar content being viewed by others
References
Bryant DM, Mostov KE (2008) From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 8:887–901
Conti MA, Adelstein RS (2008) Nonmuscle myosin II moves in new directions. J Cell Sci 121:11–18
Olguin P, Mlodzik M (2010) A new spin on planar cell polarity. Cell 142:674–676
Gniadecki R, Bang B (2003) Flotillas of lipid rafts in transit amplifying cell-like keratinocytes. J Investig Dermatol 121:522–528
Shaul PW, Anderson RG (1998) Role of plasmalemmal caveolae in signal transduction. Am J Physiol 275:L843–L851
Navarro A, Anand-Apte B, Parat MO (2004) A role for caveolae in cell migration. FASEB J 18:1801–1811
Lajoie P, Nabi IR (2010) Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol 282:135–163
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5:214.211–241.215
Rothberg G, Heuser J, Donzell W, Ying Y-S, Glenney JR, Anderson R (1992) Caveolin, a protein component of caveolae membrane coat. Cell 68:673–682
Wang Z, Tiruppathi C, Minshall RD, Malik AB (2009) Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. Nano 3:4110–4116
Lucius H, Friedrichson T, Kurzchialia T, Lewin G (2003) Identification of caveolae-like structures on the surface of intact cells using scanning force microscopy. J Membr Biol 194:97–108
Razani B, Woodman SE, Lisanti MP (2002) Caveolae: from cell biology to animal physiology. Pharmacol Rev 54:431–467
Stan RV (2005) Structure of caveolae. Biochim Biophys Acta 1746:334–348
Kenworthy A (2002) Peering inside lipid rafts and caveolae. Trends Biochem Sci 27:435–437
Kuerschner L, Ejsing CS, Ekroos K, Shevchenko A, Anderson KI, Thiele C (2005) Polyene-lipids: a new tool to image lipids. Nat Methods 2:39–45
Zheng Y, Foster J (2009) Contribution of quantitative proteomics to understanding membrane microdomains. J Lipid Res 50:1976–1985
Cialla D, März A, Böhme R, Theil F, Weber K, Schmitt M, Popp J (2012) Surface-enhanced Raman spectroscopy (SERS): progress and trends. Anal Bioanal Chem 403:27–54
Willets KA, Van Duyne P (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58:267–297
Pozzi EA, Sonntag MD, Jiang N, Klingsporn JM, Hersam MC, Van Duyne P (2013) Tip-enhanced Raman imaging: an emergent tool for probing biology at the nanoscale. ACS Nano 7:885–888
Pettinger B, Schambach P, Villagomez CJ, Scott N (2012) Tip-enhanced Raman spectroscopy: near-fields acting on a few molecules. Annu Rev Phys Chem 63:379–399
Bailo E, Deckert V (2008) Tip-enhanced Raman scattering. Chem Soc Rev 37:921–930
Richter M, Hedegaard M, Deckert-Gaudig T, Lampen P, Deckert V (2011) Laterally resolved and direct spectroscopic evidence of nanometer-sized lipid and protein domains on a single cell. Small 7:209–214
Deckert-Gaudig T, Böhme R, Freier E, Sebesta A, Merkendorf T, Popp J, Gerwert K, Deckert V (2012) Nanoscale distinction of membrane patches—a TERS study of Halobacterium salinarum. J Biophoton 5:582–591
Cialla D, Deckert-Gaudig T, Budich C, Laue M, Möller R, Naumann D, Deckert V, Popp J (2009) Raman to the limit: tip-enhanced Raman spectroscopic investigations of a single tobacco mosaic virus. J Raman Spectrosc 40:240–243
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Grande-García A, del Pozo M (2008) Caveolin-1 in cell polarization and directional migration. Eur J Cell Biol 87:641–647
Gomez-Mouton C, Lacalle RA, Mira E, Jimenez-Baranda S, Barber DF, Carrera AC, Martinez AC, Manes S (2004) Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol 164:759–768
Böhme R, Cialla D, Richter M, Rösch P, Popp J, Deckert J (2010) Biochemical imaging below the diffraction limit—probing cellular membrane related structures by tip-enhanced Raman spectroscopy (TERS). J Biophoton 3:455–461
Krafft C, Knetschke T, Siegner A, Funk RHW, Salzer R (2003) Mapping of single cells by near infrared Raman microscopy. Vib Spectrosc 32:75–83
Lamba OP, Borchman D, Sinha SK, Lal S, Yappert MC, Lou MF (1991) Structure and molecular conformation of anhydrous and aqueous sphingomyelin bilayers determined by infrared and Raman spectroscopy. J Mol Struct 248:1–24
Borchman D, Tang D, Yappert MC (1999) Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum membranes. Biospectroscopy 5:151–167
Faiman R (1977) Raman spectroscopic studies of different forms of cholesterol and its derivatives in the crystalline state. Chem Phys Lipids 18:84–104
Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC (2003) Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Investig Dermatol 120:531–541
Quest AFG, Leyton L, Párraga M (2004) Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol 82:129–144
Epand RM, Sayer BG, Epand RF (2005) Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol 345:339–350
Sonnino S, Prinetti A (2009) Sphingolipids and membrane environments for caveolin. Fed Eur Biochem Soc 583:597–606
Ramstedt B, Slotte JP (2006) Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim Biophys Acta 1758:1945–1956
Lisantti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M (1994) Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 126:111–126
Li S, Seitz R, Lisanti MP (1996) Phosphorylation of caveolin by Src tyrosine kinases. J Biol Chem 271:3863–3868
Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol 170:769–779
Li WP, Liu P, Pilcher BK, Anderson RG (2001) Cell-specific targeting of caveolin-1 to caveolae, secretory vesicles, cytoplasm or mitochondria. J Cell Sci 114:1397–1408
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW (2008) Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19:912–928
Rhim JH, Kim JH, Yeo EJ, Kim JC, Park SC (2010) Caveolin-1 as a novel indicator of wound-healing capacity in aged human corneal epithelium. Mol Med 16:527–534
Acknowledgments
We thank the Bundesministerium für Bildung und Forschung (No. 0312032B and No. 0312032C) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Single Cell Analysis with guest editors Petra Dittrich and Norbert Jakubowski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2727 kb)
Rights and permissions
About this article
Cite this article
Watkins-Mariani, M., Deckert-Gaudig, T. & Deckert, V. Label-free in vitro visualization and characterization of caveolar bulbs during stimulated re-epithelialization. Anal Bioanal Chem 406, 6993–7002 (2014). https://doi.org/10.1007/s00216-014-7998-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7998-y