Abstract
The on-line combination of comprehensive two-dimensional liquid chromatography (LC × LC) with the 2,2′-azino-bis(3-ethylbenzothiazoline)-6 sulphonic acid (ABTS) radical scavenging assay was investigated as a powerful method to determine the free radical scavenging activities of individual phenolics in natural products. The combination of hydrophilic interaction chromatography (HILIC) separation according to polarity and reversed-phase liquid chromatography (RP-LC) separation according to hydrophobicity is shown to provide much higher resolving power than one-dimensional separations, which, combined with on-line ABTS detection, allows the detailed characterisation of antioxidants in complex samples. Careful optimisation of the ABTS reaction conditions was required to maintain the chromatographic separation in the antioxidant detection process. Both on-line and off-line HILIC × RP-LC–ABTS methods were developed, with the former offering higher throughput and the latter higher resolution. Even for the fast analyses used in the second dimension of on-line HILIC × RP-LC, good performance for the ABTS assay was obtained. The combination of LC × LC separation with an on-line radical scavenging assay increases the likelihood of identifying individual radical scavenging species compared to conventional LC–ABTS assays. The applicability of the approach was demonstrated for cocoa, red grape seed and green tea phenolics.

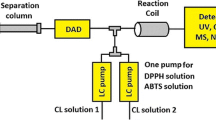
On-line HILIC×RP-LC–ABTS analysis of cocoa proanthocyanidins






Similar content being viewed by others
References
Kusznierewicz B, Piasek A, Bartoszek A, Namiesnik J (2011) Phytochem Anal 22:392–402
Szajdek A, Borowska EJ (2008) Plant Foods Hum Nutr 63:147–156
Iriti M, Faoro F (2009) In: Watson RR (ed) Complementary and alternative therapies and the aging population: an evidence-based approach. Elsevier Inc., San Diego
Shahidi F, Wanasundara PKJPD (1992) Crit Rev Food Sci Nutr 32:67–103
Koşar M, Dorman HJD, Başer KHC, Hiltunen R (2004) J Agric Food Chem 52:5004–5010
Dudonné AS, Vitrac X, Coutière P, Woillez M, Mérillon J-M (2009) J Agric Food Chem 57:1768–1774
Joubert E, Manley M, Botha M (2008) Phytochem Anal 19:169–178
Spranger I, Sun B, Mateus AM, de Freitas V, Ricardo-da-Silva JM (2008) Food Chem 108:519–532
Huang D, Ou B, Prior RL (2005) J Agric Food Chem 53:1841–1856
Magalhaes LM, Segundo MA, Reis S, Lima JLFC (2008) Anal Chim Acta 613:1–19
Karadag A, Ozcelik B, Saner S (2009) Food Anal Meth 2:41–60
Moon J-K, Shibamoto T (2009) J Agric Food Chem 57:1655–1666
Laguerre M, Decker EA, Lecomte J, Villeneuve P (2010) Curr Opin Clin Nutr Metab Care 13:518–525
Gülçin I (2012) Arch Toxicol 86:345–391
Niederländer HAG, van Beek TA, Bartasiute A, Koleva II (2008) J Chromatogr A 1210:121–134
Malherbe CJ, de Beer D, Joubert E (2012) Int J Mol Sci 13:3101–3133
Re R, Pellegrini N, Proteggente A, Apnnal A, Yang M, Rice-Evans C (1999) Free Radic Biol Med 26:1231–1237
Koleva II, Niederländer HAG, van Beek TA (2001) Anal Chem 73:3373–3381
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK (2011) J Food Comp Anal 24:1043–1048
Martysiak-Lrowska D, Wenta W (2012) Acta Sci Pol Technol Aliment 11:83–89
Li F, Zhang L-D, Li B-C, Yang J, Yu H, Wan J-B, Wang Y-T, Li P (2012) Free Radic Res 46:286–294
Bushey MM, Jorgenson JW (1990) Anal Chem 62:161–167
Davis JM, Giddings JC (1985) Anal Chem 57:2168–2177
Davis JM, Giddings JC (1985) Anal Chem 57:2178–2187
Giddings JC (1990) In: Cortes HJ (ed) Multidimensional chromatography: techniques and applications. Marcel Dekker, New York
Kalili KM, de Villiers A (2009) J Chromatogr A 1216:6274–6284
Kalili KM, de Villiers A (2010) J Sep Sci 33:853–863
Beelders T, Kalili KM, Joubert E, de Beer D, de Villiers A (2012) J Sep Sci 35:1808–1820
Kalili KM, Vestner J, Stander MA, de Villiers A (2013) Anal Chem 85:9107–9115
Kalili KM, de Villiers A (2013) J Chromatogr A 1289:58–68
Kalili KM, de Villiers A (2013) J Chromatogr A 1289:69–79
Kalili KM, Cabooter D, Desmet G, de Villiers A (2012) J Chromatogr A 1236:63–76
Kuhlmann O, Krauss G-J (1997) J Pharm Biomed Anal 16:553–559
Kucera P, Umagat H (1983) J Chromatogr 255:563–579
Lestremau F, Wu D, Szücs R (2010) J Chromatogr A 1217:4925–4933
Giddings JC (1984) Anal Chem 56:1258A–1270A
Pannala AS, Rice-Evans C (2001) Methods Enzymol 335:266–272
Kivilompolo M, Hyötyläinen T (2007) J Chromatogr A 1145:155–164
Kivilompolo M, Oburka V, Hyötyläinen T (2008) Anal Bioanal Chem 391:373–380
Dugo P, Cacciola F, Donato P, Airado-Rodríguez D, Herrero M, Mondello L (2009) J Chromatogr A 1216:7483–7487
Acknowledgments
KMK and AdV gratefully acknowledge Stellenbosch University, Sasol and the National Research Foundation (NRF, Grant 70995 to AdV) for funding. SDS and TVH gratefully acknowledge the Agency for Innovation by Science and Technology in Flanders (IWT) for financial support. Dalene de Beer is thanked for advice on the antioxidant assays and Edmund Luckay (IWBT) for the donation of the grape sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalili, K.M., De Smet, S., van Hoeylandt, T. et al. Comprehensive two-dimensional liquid chromatography coupled to the ABTS radical scavenging assay: a powerful method for the analysis of phenolic antioxidants. Anal Bioanal Chem 406, 4233–4242 (2014). https://doi.org/10.1007/s00216-014-7847-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7847-z