Abstract
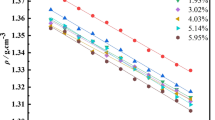
This article develops seven mathematical models to investigate how NdF3 anions coexist in LiF–NdF3 molten salt. The influence of the LiF/NdF3 mole ratio (CR) and temperature on the ion structure of the molten salt was thoroughly examined through thermodynamic calculations, and the conductivity and transference number of the ions in the molten salt were predicted using a model that has five complicated anions salt. LiF–NdF3 molten salt's ion structure analysis shows that the predominant anions in the system were NdF4−, NdF52−, NdF63−, Nd2F7−, and NdF74−, along with F−. The degree of ion aggregation in the molten salt system can be decreased by increasing the LiF content, improving the system's electrical conductivity. High temperature promotes the transformation of complex anions to simple anions in the molten salt, enhancing the transfer ability of simple anions, which is beneficial to improve the electrical conductivity of the molten salt. The calculated conductivity value agrees with the value that was measured. As the percentage of NdF3 rises, the calculated value of LiF–NdF3 molten salt conductivity falls, and the rate of decline slows. The reason for the change in conductivity was related to the ion variation.








Similar content being viewed by others
Data availability
Not applicable.
References
Gibilaro M, Remazeilles C, Massot L, Chamelot P (2022) Process Optimization to avoid perfluorocarbon emission during neodymium rare earth electrolysis in molten LiF-NdF3-Nd2O3. J Electrochem Soc 169(8):083501. https://doi.org/10.1149/1945-7111/ac8377
Kwon S, Ryu H-Y, Cho S-H, Lee J-H (2020) Effect of the electrolyte composition on the electrochemical behavior of Nd fluoride complex in a LiF-NdF3-Nd2O3 molten salt. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2020.114751
Akdeniz Z, Onem ZC, Tosi MP (2001) Structure of rare-earth/alkali halide complexes. Zeitschrift Fur Naturforschung Sect A J Phys Sci 56(11):721–724. https://doi.org/10.1515/zna-2001-1103
Hu XW, Wang ZW, Luo XD, Lu GM, Cui JZ, Chen GH (2008) Neodymium and neodymium-oxygen containing species in fluoride melts[J]. Chin Rare Earths 29(5):58–60. https://doi.org/10.16533/j.cnki.15-1099/tf.2008.05.020
Hu XW, Wang ZW, Gao BL, Shi ZN, Liu FG, Chao XZ (2010) Density and ionic structure of NdF3-LiF melts. J Rare Earths 28(04):587–590. https://doi.org/10.1016/S1002-0721(09)60159-9
Hu XW, Wang ZW, Gao BL, Shi ZN, Liu FG, Bao MG (2010) Identification of structural entities in NdF3-LiF melts with cryoscopic method. Trans Nonferrous Metals Soc China 20(12):2387–2391. https://doi.org/10.1016/s1003-6326(10)60659-0
Dracopoulos V, Gilbert B, Papatheodorou G (1998) Vibrational modes and structure of lanthanide fluoride–potassium fluoride binary melts LnF3–KF (Ln= La, Ce, Nd, Sm, Dy, Yb). J Chem Soc, Faraday Trans 94(17):2601–2604. https://doi.org/10.1039/A802812E
Stefanidaki E, Photiadis GM, Kontoyannis CG, Vik AF, Østvold T (2002) Oxide solubility and Raman spectra of NdF3–LiF–KF–MgF2–Nd2O3 melts. J Chem Soc, Dalton Trans 11:2302–2307. https://doi.org/10.1039/b111563b
Zhu X, Sun S, Sun T, Liu C, Tu G, Zhang J (2020) Electrical conductivity of REF3-LiF (RE=La and Nd) molten salts. J Rare Earths 38(6):676–682. https://doi.org/10.1016/j.jre.2019.04.017
Tan M, Li T, Shang B, Cui H (2020) Quantum chemical prediction of the spectroscopic properties and ionic composition of the molten NaF-AlF3 salts. J Mol Liq 317:113937. https://doi.org/10.1016/j.molliq.2020.113937
Tan M, Li T, Yan J, Shang Z (2020) Thermodynamic investigation on ionic structure and electrical conductivity of molten naf-alf3 salts. J Electrochem Soc 167(2):023503. https://doi.org/10.1149/1945-7111/ab69f1
Lu T, Chen Q (2021) Shermo: a general code for calculating molecular thermochemistry properties. Comput Theor Chem 1200:113249. https://doi.org/10.1016/j.comptc.2021.113249
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. (2016) Gaussian 16 Rev. A.03. In. Wallingford, CT
Bale CW, Chartrand P, Degterov SA, Eriksson G, Hack K, Ben Mahfoud R et al (2002) FactSage thermochemical software and databases. Calphad Comput Coupling Phase Diagr Thermochem 26(2):189–228. https://doi.org/10.1016/s0364-5916(02)00035-4
Lu X (1997) Application of the Nernst-Einstein equation to concrete. Cem Concr Res 27(2):293–302. https://doi.org/10.1016/s0008-8846(96)00200-1
Mariani RD, Vaden D (2010) Modeled salt density for nuclear material estimation in the treatment of spent nuclear fuel. J Nucl Mater 404(1):25–32. https://doi.org/10.1016/j.jnucmat.2010.06.022
Janz GJ (1967) Molten salts handbook. Molten Salts Handbook, pp 39–51
Hara S, Ikemiya N, Ogino K (1990) Surface-tension and density of molten rare-earth-metal fluorides. Denki Kagaku 58(2):156–161. https://doi.org/10.2355/tetsutohagane1955.64.5_523
Edward JT (1970) Molecular volumes and the Stokes-Einstein equation. J Chem Educ 47(4):261. https://doi.org/10.1021/ed047p261
Lu GM, Zhang RQ (1990) Mathematical model of NdF3-LiF system fused salt density and the effect of BaF2 additive on it [J]. Nonferrous Metals Metall 3:35–39
Zhang J, Lu T (2021) Efficient evaluation of electrostatic potential with computerized optimized code. Phys Chem Chem Phys 23(36):20323–20328. https://doi.org/10.1039/d1cp02805g
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Harari D, Lantelme F, Chemla M (2017) Mesure des coefficients de diffusion de 22Na et de 18F dans les bains de fluorures de sodium et d’aluminium. J Chem Phys 66(8):1286–1291. https://doi.org/10.1051/jcp/196966s21286
Janz GJ, Bansal NP (1982) Molten salts data: diffusion coefficients in single and multi-component salt systems. J Phys Chem Ref Data 11(3):505–693. https://doi.org/10.1063/1.555665
Zhen TC, Ren YH, Mao YW (2000) A study on the viscosity of NdF3-LiF-Nd2O3 system. Translated from Chinese by Que Z. Chinese Rare Earth 21(6):33–36. https://doi.org/10.16533/j.cnki.15-1099/tf.2000.06.010
Thoma RE, Brunton GD, Penneman RA, Keenan TK (1970) Equilibrium relations and crystal structure of lithium fluorolanthanate phases. Inorg Chem 9(5):1096–1101. https://doi.org/10.1021/ic50087a019
Nazmutdinov RR, Zinkicheva TT, Vassiliev SY, Glukhov DV, Tsirlina GA, Probst M (2010) A spectroscopic and computational study of Al (III) complexes in sodium cryolite melts: Ionic composition in a wide range of cryolite ratios. Spectrochim Acta Part A Mol Biomol Spectrosc 75(4):1244–1252. https://doi.org/10.1016/j.saa.2009.12.035
Gu JJ, Fan CM, Tian L, Xie G, Yang N, Li B (2021) Effect of Fe2O3 on electrical conductivity of NdF3-LiF-Nd2O3. Non-Ferrous Eng 11(4):47–54. https://doi.org/10.3969/j.issn.2095-1744.2021.04.008
Chen SM, Liao CF, Lin JY, Cai BQ, Wang X, Jiao YF (2019) Electrical conductivity of molten LiF–DyF3–Dy2O3–Cu2O system for Dy–Cu intermediate alloy production. Int J Miner Metall Mater 26(6):701–709. https://doi.org/10.1007/s12613-019-1775-z
Korenko M, Krishnan D, Šimko F, Ambrová M, Szatmáry L (2022) Electrical conductivity of the molten systems of (LiF–CaF2) eut–NdF3 and (LiF–NaF) eut–NdF3. J Mol Liq 365:120012. https://doi.org/10.1016/j.molliq.2022.120012
Kan H, Wang Z, Ban Y, Shi Z, Qiu Z (2007) Electrical conductivity of Na3AlF6-AlF3-Al2O3-CaF2-LiF (NaCl) system electrolyte. Trans Nonferrous Metals Soc China 17(1):181–186. https://doi.org/10.1016/s1003-6326(07)60069-7
Koishi T, Kawase S, Tamaki S (2002) A theory of electrical conductivity of molten salt. J Chem Phys 116(7):3018–3026. https://doi.org/10.1063/1.1436476
Salanne M, Simon C, Turq P, Madden PA (2007) Conductivity−viscosity−structure: unpicking the relationship in an ionic liquid. J Phys Chem B 111(18):4678–4684. https://doi.org/10.1021/jp067073a
Hu X. W. Study on ionic structure and its application of NdF3-LiF-Nd2O3 system melts. Northeastern University; 2008.
Acknowledgements
All authors acknowledge the funding from the National Natural Science Foundation of China (grant No.52174335 and 52074134).
Funding
This study was funded by National Natural Science Foundation of China (52174335) and National Natural Science Foundation of China (52074134).
Author information
Authors and Affiliations
Contributions
ZF contributed to the conceptualization, methodology, formal analysis, investigation, writing–original draft, and visualization. CL was involved in the methodology, review and editing, visualization, and funding acquisition. XW contributed to the supervision, resources, and funding acquisition. GD assisted in the methodology, formal analysis, and investigation. XZ performed the formal analysis and investigation. LQ was involved in the formal analysis, investigation, and visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Z., Liao, C., Wang, X. et al. Thermodynamic investigation on ion structure and conductivity of LiF–NdF3 molten salt. Theor Chem Acc 142, 115 (2023). https://doi.org/10.1007/s00214-023-03056-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03056-y