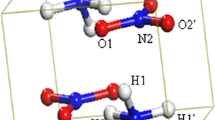
Abstract
With the oceans covering more than 70% of the Earth’s surface, sea spray aerosol particles contribute significantly to the Earth’s radiation budget and serve as seeds for cloud formation and impact various biogeochemical cycles and ecosystems. The sea spray aerosol particles are being the mixture of inorganic sea salt and organic substances and are one of the largest sources of natural aerosol particles on Earth’s atmosphere. Sodium \( \left( {{\text{Na}}^{ + } } \right) \) and lithium \( \left( {{\text{Li}}^{ + } } \right) \) ions are common constituents of ocean waters and found in abundance in sea spray aerosol particles. The gaseous ammonia is the most abundant alkaline gas in the marine troposphere and plays a vital role in forming marine aerosol particles. In this study, the structural characteristics and thermodynamic properties of sodium ion and lithium ion hydrated clusters were analyzed. Ammonia containing sodium and lithium ion hydrated clusters have also been investigated. Rayleigh scattering properties of these clusters have been investigated systematically. All these properties are obtained by using different levels of density functional theory. We find that sodium and lithium ions can form stable clusters from the gas phase (i.e., nucleation). Furthermore, sodium and lithium ion hydrated aerosol formation are enhanced substantially by the presence of ammonia. Our results suggest that the Rayleigh scattering intensity shows quadratic growth with the increase in the cluster size. The results also indicate that a single ammonia molecule in cluster results in increased scattering intensity of the ammonia containing sodium and lithium ion hydrated clusters. We computed Rayleigh scattering intensities for our studied clusters at various wavelengths (∞, 700, 600, 500, and 400 nm). These wavelengths follow the same growth pattern, with 400 nm having a substantial increase of Rayleigh scattering intensities.








Similar content being viewed by others
References
Aerosols and Incoming Sunlight (Direct Effects). [Internet]. NASA. Accessed 13 June 2019
Haywood J, Boucher O (2000) Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: a review. Rev Geophys 38:513–543
Kulmala M, Riipinen I, Sipilä M, Manninen HE, Petäjä T, Junninen H et al (2007) Toward direct measurement of atmospheric nucleation. Science 318:89–92
Sipilä M, Lehtipalo K, Kulmala M, Petäjä T, Junninen H, Aalto PP et al (2008) Applicability of condensation particle counters to measure atmospheric clusters. Atmos Chem Phys 8:4049–4060
Lehtipalo K, Sipila M, Riipinen I, Nieminen T, Kulmala M (2009) Analysis of atmospheric neutral and charged molecular clusters in boreal forest using pulse-height CPC. Atmos Chem Phys 9:4177–4184
Manninen HE, Petäjä T, Asmi E, Riipinen I, Nieminen T, Mikkilä J et al (2009) Long-term field measurements of charged and neutral clusters using neutral cluster and air ion spectrometer (NAIS). Boreal Environ Res 14:591–605
Brasseur GP, Solomon S (2005) Aeronomy of the middle atmosphere. In: Hamilton LAMK (ed) Aeronomy of the middle atmosphere. 32. Third revised and enlarged edition ed. Springer, Boulder, Co, pp 177–80
Zhang R (2010) Getting to the critical nucleus of aerosol formation. Science 328:1366–1367
Zhang R, Khalizov A, Wang L, Hu M, Xu W (2012) Nucleation and growth of nanoparticles in the atmosphere. Chem Rev 112:1957–2011
Kulmala M (2003) How particles nucleate and grow. Science 302:1000–1001
Zhang R, Suh I, Zhao J, Zhang D, Fortner EC, Tie X et al (2004) Atmospheric new particle formation enhanced by organic acids. Science 304:1487–1490
Kulmala M, Petäjä T, Nieminen T, Sipilä M, Manninen HE, Lehtipalo K et al (2012) Measurement of the nucleation of atmospheric aerosol particles. Nat Protoc 7:1651–1667
Kulmala M, Kontkanen J, Junninen H, Lehtipalo K, Manninen HE, Nieminen T et al (2013) Direct observations of atmospheric aerosol nucleation. Science 339:943–946
Sipilä M, Berndt T, Petäjä T, Brus D, Vanhanen J, Stratmann F et al (2010) The role of sulfuric acid in atmospheric nucleation. Science 327:1243–1246
Kirkby J, Curtius J, Almeida J, Dunne E, Duplissy J, Ehrhart S et al (2011) Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476:429–433
Kurtén T, Loukonen V, Vehkamäki H, Kulmala M (2008) Amines are likely to enhance neutral and ion-induced sulfuric acid-water nucleation in the atmosphere more effectively than ammonia. Atmos Chem Phys 8:4095–4103
Ortega IK, Kupiainen O, Kurtén T, Olenius T, Wilkman O, McGrath MJ et al (2012) From quantum chemical formation free energies to evaporation rates. Atmos Chem Phys 12:225–235
Zhao J, Khalizov A, Zhang R, McGraw R (2009) Hydrogen-bonding interaction in molecular complexes and clusters of aerosol nucleation precursors. J Phys Chem A 113:680–689
Andreae MO (2013) The aerosol nucleation puzzle. Science 339:911–912
Riccobono F, Schobesberger S, Scott CE, Dommen J, Ortega IK, Rondo L et al (2014) Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science 344:717–721
Ehn M, Thornton JA, Kleist E, Sipila M, Junninen H, Pullinen I et al (2014) A large source of low-volatility secondary organic aerosol. Nature 506:476–479
Kirkby J, Duplissy J, Sengupta K, Frege C, Gordon H, Williamson C et al (2016) Ion-induced nucleation of pure biogenic particles. Nature 533:521–526
Buszek RJ, Francisco JS, Anglada JM (2011) Water effects on atmospheric reactions. Int Rev Phys Chem 30:335–369
Ding G, Laasonen K, Laaksonen A (2003) Two sulfuric acids in small water clusters. J Phys Chem A 107:8648–8658
Ding G, Laasonen K (2004) Partially and fully deprotonated sulfuric acid in H2SO4(H2O)n (n = 6 − 8) clusters. Chem Phys Lett 390:307–313
Kildgaard JV, Mikkelsen KV, Bilde M, Elm J (2018) Hydration of atmospheric molecular clusters: a new method for systematic configurational sampling. J Phys Chem A 122:5026–5036
Zollner JH, Glasoe WA, Panta B, Carlson KK, McMurry PH, Hanson DR (2012) Sulfuric acid nucleation: power dependencies, variation with relative humidity, and effect of bases. Atmos Chem Phys 12:4399–4411
Glasoe WA, Volz K, Panta B, Freshour N, Bachman R, Hanson DR et al (2015) Sulfuric acid nucleation: an experimental study of the effect of seven bases. J Geophys Res Atmos 120:1933–1950
Woodcock AH (1953) Salt nuclei in marine air as a function of altitude and wind force. J Meteorol 10:362–371
Knelman F, Dombrowski N, Newitt DM (1954) Mechanism of the bursting of bubbles. Nature 173:261
Spiel DE (1998) On the births of film drops from bubbles bursting on seawater surfaces. J Geophys Res Oceans 103:24907–24918
Lewis ER, Schwartz SE (2004) Sea salt aerosol production: mechanisms, methods, measurements and models—a critical review. Am Geophys Union
Haywood JM, Ramaswamy V, Soden BJ (1999) Tropospheric aerosol climate forcing in clear-sky satellite observations over the oceans. Science 283:1299–1303
Andreae MO, Rosenfeld D (2008) Aerosol–cloud–precipitation interactions Part 1 The nature and sources of cloud-active aerosols. Earth-Sci Rev 89:13–41
Lihavainen H, Kerminen VM, Komppula M, Hyvärinen AP, Laakia J, Saarikoski S et al (2008) Measurements of the relation between aerosol properties and microphysics and chemistry of low-level liquid water clouds in Northern Finland. Atmos Chem Phys 8:6925–6938
George SK, Nair PR, Parameswaran K, Jacob S, Abraham A (2008) Seasonal trends in chemical composition of aerosols at a tropical coastal site of India. J Geophys Res Atmos 113:D16209
Metzger S, Mihalopoulos N, Lelieveld J (2006) Importance of mineral cations and organics in gas-aerosol partitioning of reactive nitrogen compounds: case study based on MINOS results. Atmos Chem Phys 6:2549–2567
Millero FJ (2005) Chemical oceanography. CRC Press, Boca Raton
Xiao H-W, Xiao H-Y, Shen C-Y, Zhang Z-Y, Long A-M (2018) Chemical composition and sources of marine aerosol over the western north pacific ocean in winter. Atmosphere 9:298
Cardoso J, Almeida SM, Nunes T, Almeida-Silva M, Cerqueira M, Alves C et al (2018) Source apportionment of atmospheric aerosol in a marine dusty environment by ionic/composition mass balance (IMB). Atmos Chem Phys 18:13215–13230
Xiao HW, Xiao HY, Luo L, Shen CY, Long AM, Chen L et al (2017) Atmospheric aerosol compositions over the South China Sea: temporal variability and source apportionment. Atmos Chem Phys 17:3199–3214
Lyons WB, Welch KA (1997) Lithium in waters of a polar desert. Geochim Cosmochim Acta 61:4309–4319
Quinn PK, Charlson RJ, Zoller WH (1987) Ammonia, the dominant base in the remote marine troposphere: a review. Tellus B 39:413–425
Dzidic I, Kebarle P (1970) Hydration of the alkali ions in the gas phase. Enthalpies and entropies of reactions M+(H2O)n-1 + H2O = M+(H2O)n. J Phys Chem 74:1466–1474
Feller D, Glendening ED, Kendall RA, Peterson KA (1994) An extended basis set ab initio study of Li+(H2O)n, n = 1–6. J Chem Phys 100:4981–4997
Feller D, Glendening ED, Woon DE, Feyereisen MW (1995) An extended basis set ab initio study of alkali metal cation–water clusters. J Chem Phys 103:3526–3542
Kim J, Lee S, Cho SJ, Mhin BJ, Kim KS (1995) Structures, energetics, and spectra of aqua-sodium(I): thermodynamic effects and nonadditive interactions. J Chem Phys. 102:839–849
Rodgers MT, Armentrout PB (1997) Collision-induced dissociation measurements on Li + (H2O)n, n = 1–6: the first direct measurement of the Li + −OH2 bond energy. J Phys Chem A 101:1238–1249
Hashimoto K, Kamimoto T (1998) Theoretical study of microscopic solvation of lithium in water clusters: neutral and cationic Li(H2O)n (n = 1–6 and 8). J Am Chem Soc 120:3560–3570
Lukyanov SI, Zidi ZS, Shevkunov SV (2003) Study of aqvist’s ion–water interaction model in na+–water clusters: free energy and structure. J Mol Struct: THEOCHEM. 623:221–236
Li X, Yang Z-Z (2005) Study of lithium cation in water clusters: based on atom-bond electronegativity equalization method fused into molecular mechanics. J Phys Chem A 109:4102–4111
Zidi ZS (2012) On the stability of ion water clusters at atmospheric conditions: open system Monte Carlo simulation. J Chem Phys. 137:124107
Elm J, Bilde M, Mikkelsen KV (2012) Assessment of density functional theory in predicting structures and free energies of reaction of atmospheric prenucleation clusters. J Chem Theory Comput 8:2071–2077
Elm J, Bilde M, Mikkelsen KV (2013) Assessment of binding energies of atmospherically relevant clusters. Phys Chem Chem Phys 15:16442–16445
Leverentz HR, Siepmann JI, Truhlar DG, Loukonen V, Vehkamäki H (2013) Energetics of atmospherically implicated clusters made of sulfuric acid, ammonia, and dimethyl amine. J Phys Chem A 117:3819–3825
Bork N, Du L, Kjaergaard HG (2014) Identification and characterization of the HCl–DMS gas phase molecular complex via infrared spectroscopy and electronic structure calculations. J Phys Chem A 118:1384–1389
Zhao Y, Truhlar DG (2008) The M06 Suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 120:215–241
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 98:5648–5652
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Kildgaard JV, Mikkelsen KV, Bilde M, Elm J (2018) Hydration of atmospheric molecular clusters II: organic acid-water clusters. J Phys Chem A 122:8549–8556
Elm J, Mikkelsen KV (2014) Computational approaches for efficiently modelling of small atmospheric clusters. Chem Phys Lett 615:26–29
Myllys N, Elm J, Kurtén T (2016) Density functional theory basis set convergence of sulfuric acid-containing molecular clusters. Comput Theor Chem. 1098:1–12
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16 revision, A03 edn. Gaussian Inc, Wallingford
Elm J, Norman P, Bilde M, Mikkelsen KV (2014) Computational study of the rayleigh light scattering properties of atmospheric pre-nucleation clusters. Phys Chem Chem Phys 16:10883–10890
Peng X-Q, Liu Y-R, Huang T, Jiang S, Huang W (2015) Interaction of gas phase oxalic acid with ammonia and its atmospheric implications. Phys Chem Chem Phys 17:9552–9563
Wenger M, Armbruster T (1991) Crystal chemistry of lithium; oxygen coordination and bonding. Eur J Mineral 3:387–399
Wood RM, Palenik GJ (1999) Bond valence sums in coordination chemistry sodium − oxygen complexes. Inorg Chem 38:3926–3930
Yamaji K, Makita Y, Watanabe H, Sonoda A, Kanoh H, Hirotsu T et al (2001) Theoretical estimation of lithium isotopic reduced partition function ratio for lithium ions in aqueous solution. J Phys Chem A 105:602–613
Watanabe H, Yamaji K, Sonoda A, Makita Y, Kanoh H, Ooi K (2003) Theoretical estimation of the solvent effect of the lithium isotopic reduced partition function ratio. J Phys Chem A 107:7832–7844
Funding
This work was supported by the Council of Scientific and Industrial Research of India (Grant No. 01 (2890)/17/EMR-11). The author is also thankful to the Indian Institute of Technology Patna for financial support and research facilities at IIT Patna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors reported no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pal, J., Teja, P.S. & Subramanian, R. Sodium and lithium ions in aerosol: thermodynamic and rayleigh light scattering properties. Theor Chem Acc 139, 173 (2020). https://doi.org/10.1007/s00214-020-02683-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02683-z