Abstract
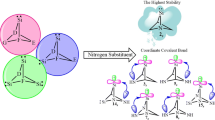
Following our quest for novel carbenes, effects of substituting 1 to 5 nitrogen atoms on the stability and reactivity of singlet (s) and triplet (t) forms of 7-boratricyclo[1,1,1,01,7,07,3,07,5]hexa-2-carbylenes (1–20) are compared and contrasted, at B3LYP/aug-cc-pvtz level of theory. All species appear as ground state minima on their energy surface, for showing no negative force constant. Singlets (1s–20s) are ground states and more stable than their corresponding triplets (1t–20t). Reactivity of the species (1s–20s vs. 1t–20t) is discussed in terms of isodesmic reactions, considering nucleophilicity (N), electrophilicity (ω), and heat of hydrogenation. As well as, the addition of nitrogen atoms decreased nucleophilicity (N), while increasing electrophilicity (ω). Despite the enormous steric strain involved in their cubic structures, the most stable scrutinized carbenes appear to be singlet 1,4,5-triaza-7-boratricyclo[1,1,1,01,7,07,3,07,5]hexa-2-carbylene (13) for showing the highest value of ΔEs–t. Such higher stabilization is attributed to a coordinate covalent bond observed between the carbenic center and the boron atom. This study offers new insights into the chemistry of these exotic tricyclic shaped carbenes.

Similar content being viewed by others
References
Kassaee MZ, Ghambarian M, Musavi SM, Shakib FA, Momeni MR (2009) J Phys Org Chem 22:919–924
Nesterov V, Reiter D, Bag P, Frisch P, Holzner R, Porzelt A, Inoue S (2018) Chem Rev 118:9678–9842
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) Nature 510:485–496
Liu M, Li Q, Li W, Cheng J (2016) Struct Chem 28(3):823–831
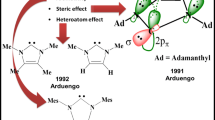
Arduengo J III, Dias HVR, Dixon DA, Harlow RL, Klooster WT, Koetzle TF (1994) J Am Chem Soc 116:6812–6822
Aysin RR, Bukalov SS, Leites LA, Zabula AV (2017) Dalton Trans 46:8774–8781
Gonzalez C, Restrepo-Cossio A, Marquez M, Wiberg KB (1996) J Am Chem Soc 118:5408–5411
Cabeza JA, García-Álvarez P, Pérez-Carreño E, Polo D (2014) Inorg Chem 53:8735–8741
Kassaee MZ, Shakib FA, Momeni MR, Ghambarian M, Musavi SM (2010) J Org Chem 75:2539–2545
Abu-Saleh A-AA, Almatarneh MH, Poirier RA (2018) Chem Phys Lett 698:36–40
Kirmse W (2004) Angew Chem Int Ed 43(14):1767–1769
Akbari A, Golzadeh B, Arshadi S, Kassaee MZ (2015) RSC Adv 5:43319–43327
Kirilchuk AA, Rozhenko AB, Leszczynski J (2017) Comp Theor Chem 1103:83–91
Arduengo AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361–363
Schuster O, Yang L, Raubenheimer HG, Albrecht M (2009) Chem Rev 109:3445–3478
Kato T, Maerten E, Baceiredo A (2010) Organomet Chem 30:131–147
Lee TJ, Bunge A, Schaefer HF (1985) J Am Chem Soc 107(1):137–142
Despagnet-Ayoub E, Grubbs RH (2004) J Am Chem Soc 126:10198–10199
Despagnet-Ayoub E, Grubbs RH (2005) Organometallics 24:338–340
Hahn FE, Jahnke MC (2008) Angew Chem Int Ed 47:3122–3172
Enders D, Breuer K, Raabe G, Runsink J, Teles JH, Melder J, Ebel K, Brode S (1995) Angew Chem Int Ed 34(9):1021–1023
Krahulic KE, Tuononen HM, Parvez M, Roesler R (2009) J Am Chem Soc 131:5858–5865
Mendoza-Espinosa D, Donnadieu B, Bertrand G (2010) J Am Chem Soc 132:7264–7265
Alder RW, Blake ME, Bortolotti C, Bufali S, Butts CP, Linehan E, Oliva JM, Orpen AG, Quayle M (1999) Chem Commun 241–242
Mayr M, Wurst K, Ongania K-H, Buchmeiser MR (2004) Chem A Eur J 10:1256–1266
Driess M, Yao S, Brym M, Wüllen CV (2006) Angew Chem Int Ed 2006(45):4349–4352
Bazinet P, Ong T-G, O’Brien JS, Lavoie N, Bell E, Yap GPA, Korobkov I, Richeson DS (2007) Organometallics 26:2885–2895
Gómez-Bujedo S, Alcarazo M, Pichon C, Alvarez E, Fernández R, Lassaletta JM (2007) Chem Commun 1180–1182
Lai CH (2013) J Mol Model 19(12):5523–5532
Heikki TM, Roesler R, Jason LD, Ragogna PJ (2007) Inorg Chem 46:10693–10706
Alder RW, Blake ME, Bortolotti C, Bufali S, Butts CP, Linehan E, Oliva JM, Orpen AG, Quayle M (1999) J Chem Commum 3:241
Zhang X, Wang K, Niu T (2014) J Struct Chem 26(2):599–606
Chu Q, Makhlouf Brahmi M, Solovyev A, Ueng S-H, Curran DP, Malacria M, Lacôte E (2009) Chem Eur J 15(47):12937–12940
Curran DP, Solovyev A, Makhlouf Brahmi M, Fensterbank L, Malacria M (2011) Angew Chem Int Ed 50:10294–10317
Makhouf Brahmi M, Monot J, Desage-El Murr M, Curran DP, Fensterbank L (2010) J Org Chem 75:6983–6985
Patra SG (2019) Comput Theor Chem 1164:112557
Ueng SH, Fensterbank L, Lacote E, Malacria M, Curran DP (2010) Org Lett 12:3002–3005
Monot J, Makhlouf Brahmi M, Ueng SH, Curran DP, Malacria M, Fensterbank L, Lacote E (2009) Org Lett 11:4914–4917
Horn M, Mayr H, Lacote E, Merling E, Deaner J, Wells S, McFadden T, Curran DP (2012) Org Lett 14:82–85
Pan X, Lacote E, Lalevee J, Curran DP (2012) J Am Chem Soc 134:5669–5674
Lindsay DM, McArthuR D (2010) Chem Commun 46:2474–2476
Ogawa A, Curran DP (1997) J Org Chem 62:450–451
Tehfe MA, MakhloufBrahmi M, Fouassier JP, Curran DP, Malacria M, Fensterbank L, Lacote E, Lalevee J (2010) Macromolecules 43:2261–2267
Tehfe MA, Monot J, Malacria M, Fensterbank L, Fouassier JP, Curran DP, Lacote E, Lalevee J (2012) ACS Macro Lett 1:92–95
Sundermann A, Reiher M, Schoeller WW (1998) Eur J Inorg Chem 3:305–310
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Schleyer PR, Maerker C, Dransfeld A, Jiao H, Hommes NJRE (1996) J Am Chem Soc 118:6317–6318
Domingo LR, Chamorro E, Perez P (2008) J Org Chem 73:4615–4624
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Kim K, Jordan KD (1994) J Phys Chem 98:10089–10094
Hehre WJ, Radom L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Doering WE, Hoffmann AK (1954) J Am Chem Soc 76:6162–6165
Hoffmann R, Schleyer PR, Schaefer HF (2008) Angew Chem Int Ed 47:7164–7167
Haerizade BN, Kassaee MZ, Zandi H, Koohi M, Ahmadia AA (2014) J Phys Org Chem 27:902–908
Martin D, Baceiredo A, Gornitzka H, Schoeller WW, Bertrand G (2005) Angew Chem Int Ed 44:1700–1703
Hehre WJ, Ditchfield R, Radom L, Pople JA (1970) J Am Chem Soc 92:4796–4801
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged.
Funding
Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abedini, N., Kassaee, M.Z. & Cummings, P.T. Effects of nitrogen atoms on the stability and reactivity of tricyclic boracarbenes by DFT. Theor Chem Acc 139, 146 (2020). https://doi.org/10.1007/s00214-020-02659-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02659-z