Abstract
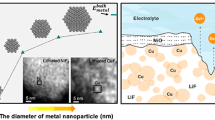
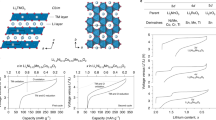
The successful use of metal oxides as conversion anodes in Li-ion batteries invokes the formation and subsequent reductive decomposition of Li2O. Given the standard reduction potential of Li/Li+ couple, the reduction of Li2O to Li is a thermodynamic challenge. This work investigates the interaction of Li+ ions with a Cu2O matrix computationally using the first principles-based DFT + U methodology. Alloying of Cu and Li takes place more readily than Li2O formation in the early part of the charge cycle. Li2O formation is predicted in the later part of the charge cycle. We attribute the capacity fading observed in oxide conversion anodes to the irreversible accumulation of Li2O and the reversible charge storage capacity delivered by the conversion anodes to alloying. This work indicates that reversible alloying plays a far greater role in the charge–discharge process than is generally acknowledged.







Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Nature 414:359–367
Goodenough JB, Park KS (2013) J Am Chem Soc 135:1167–1176
Tarascon JM, Armand M (2008) Nature 451:652–657
Scrosati B, Garche J (2010) J Power Sour 195:2419–2490
Whittingham MS (2004) Chem Rev 104:4271–4301
Goriparti S, Miele E, Angelis FD, Fabrizio ED, Zaccaria RP, Capiglia C (2014) J Power Sour 257:421–443
Li CC, Wang YW (2013) J Power Sour 227:204–210
Croguennec L, Palacin MR (2015) J Am Chem Soc 137:3140–3156
Hou J, Shao Y, Ellis MW, Moore RB, Yi B (2011) Phys Chem Chem Phys 13:15384–15402
Landi BJ, Ganter MJ, Cress CD, DiLeo RA, Raffaelle RP (2009) Energy Environ Sci 2:638–654
Zhou H, Zhu S, Hibino M, Honma I, Ichihara M (2003) Adv Mater 15:2107–2111
Persson K, Sethuraman VA, Hardwick LJ, Hinuma Y, Meng YS, van der Ven A, Srinivasan V, Kostecki R, Ceder G (2010) J Phys Chem Lett 1:1176–1180
Kaskhedikar NA, Maier J (2009) Adv Mater 21:2664–2680
Casimir A, Zhang H, Ogoke O, Amine JC, Lu J, Wu G (2016) Nano Energy 27:359–376
Hwang IS, Kim JC, Seo SD, Lee S, Lee JH, Kim DW (2012) Chem Commun 48:7061–7063
Li H, Wang Z, Chen L, Huang X (2009) Adv Mater 21:4593–4607
Ji L, Lin Z, Alcoutlabi M, Zhang X (2011) Energy Environ Sci 4:2682–2699
Boyanov S, Annou K, Villevieille C, Pelosi M, Zitoun D, Monoconduit L (2008) Ionics 14:183–190
Lai CH, Lu MY, Chen LJ (2012) J Mater Chem 22:19–30
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nature 407:496–499
Badway F, Plitz I, Grugeon S, Laruelle S, Dollé M, Gozdz AS, Tarascon JM (2002) Electrochem Solid-State Lett 5(6):A115–A118
Cao K, Jin T, Li Y, Jiao L (2017) Mater Chem Front 1:2213–2242
Belliard F, Irvine JTS (2001) J Power Sour 97–98:219–222
Wang L, Maxisch T, Ceder G (2006) Phys Rev B 73:195107–195112
Scanlon DO, Morgan BJ, Watson GW (2009) J Chem Phys 131:124703–125710
Mishra AK, Roldan A, de Leeuw NH (2016) J Phys Chem C 120:2198–2214
Tahir D, Tougaard S (2012) J Phys Condens Mater 24:175002–175007
Acknowledgements
GKK acknowledges the Council of Scientific and Industrial Research, Government of India (GOI), for the award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiran, G.K., Periyasamy, G. & Kamath, P.V. Role of alloying in Cu2O conversion anode for Li-ion batteries. Theor Chem Acc 138, 23 (2019). https://doi.org/10.1007/s00214-018-2412-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2412-z