Abstract
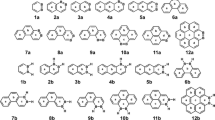
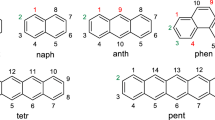
The Clar π-sextet rule was formulated as a tool to qualitatively assign the local aromatic character of six-membered rings in benzenoid species. This simple rule has been widely validated both experimentally and theoretically. In 1984, Glidewell and Lloyd reported an extension of this rule to polycyclic conjugated hydrocarbons having rings with any even number of carbon atoms in their structure. In this work, we assess the validity of the Glidewell–Lloyd extension in 69 polycyclic conjugated hydrocarbons composed of different combinations of four-, six-, and eight-membered rings. Our results support the validity of this extension with some exceptions that are discussed. Finally, a minor modification to the rule is proposed.






Similar content being viewed by others
References
Hückel E (1931) Quantentheoretische Beiträge zum Benzolproblem I: Die Elektronenkonfiguration des Benzols und verwandter Verbindungen. Z Phys 70:104–186
Hückel E (1931) Quanstentheoretische Beiträge zum Benzolproblem II: Quantentheorie der induzierten Polaritäten. Z Phys 72:310–337
Hückel E (1932) Quantentheoretische Beiträge zum Problem der aromatischen und ungesättigten Verbindungen: III. Z Phys 76:628–648
Hückel E (1937) The theory of unsaturated and aromatic compounds. Z Elektrochem 43(752–788):827–849
Clar E (1972) The aromatic sextet. Wiley, New York
Solà M (2013) Forty years of Clar’s aromatic π-sextet rule. Front Chem 1:22
Portella G, Poater J, Bofill JM, Alemany P, Solà M (2005) Local aromaticity of [n]Acenes, [n]Phenacenes, and [n]Helicenes (n = 1–9). J Org Chem 70:2509–2521
Glidewell C, Lloyd D (1984) MNDO study of bond orders in some conjugated bi- and tri-cyclic hydrocarbons. Tetrahedron 40:4455–4472
Vol’pin ME (1960) Non-benzenoid aromatic compounds and the concept of aromaticity. Russ Chem Rev 29:129–160
Randić M (2003) Aromaticity of polycyclic conjugated hydrocarbons. Chem Rev 103:3449–3605
Ginsburg D (1959) Non-benzenoid aromatic compounds. Interscience Publishers Inc., New York
Breslow R (2014) Novel aromatic and antiaromatic systems. Chem Rec 14:1174–1182
Miyoshi H, Nobusue S, Shimizu A, Tobe Y (2015) Non-alternant non-benzenoid kekulenes: the birth of a new kekulene family. Chem Soc Rev 44:6560–6577
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02, Gaussian Inc, Pittsburgh
Becke AD (1993) Density-functional thermochemistry. III: the role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25: supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Poater J, Duran M, Solà M, Silvi B (2005) Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem Rev 105:3911–3947
Feixas F, Matito E, Poater J, Sola M (2015) Quantifying aromaticity with electron delocalisation measures. Chem Soc Rev 44:6434–6451
Bultinck P, Ponec R, Van Damme S (2005) Multicenter bond indices as a new measure of aromaticity in polycyclic aromatic hydrocarbons. J Phys Org Chem 18:706–718
Matito E, Duran M, Solà M (2005) The aromatic fluctuation index (FLU): a new aromaticity index based on electron delocalization. J Chem Phys 122:014109
Kruszewski J, Krygowski TM (1972) Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett 13:3839–3842
Krygowski TM (1993) Crystallographic studies of inter- and intra-molecular interactions reflected in benzenoid hydrocarbons: nonequivalence of indices of aromaticity. J Chem Inf Comput Sci 33:70–78
Giambiagi M, de Giambiagi MS, dos Santos CD, de Figueiredo AP (2000) Multicenter bond indices as a measure of aromaticity. Phys Chem Chem Phys 2:3381–3392
Bader RFW, Stephens ME (1975) Spatial localization of the electronic pair and number distributions in molecules. J Am Chem Soc 97:7391–7399
Fradera X, Austen MA, Bader RFW (1999) The Lewis model and beyond. J Phys Chem A 103:304–314
Fradera X, Poater J, Simon S, Duran M, Solà M (2002) Electron-pairing analysis from localization and delocalization indices in the framework of the atoms-in-molecules theory. Theor Chem Acc 108:214–224
Matito E, Poater J, Solà M, Duran M, Salvador P (2005) Comparison of the AIM delocalization index and the Mayer and fuzzy atom bond orders. J Phys Chem A 109:9904–9910
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon, Oxford
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Matito E (2006) ESI-3D: electron sharing indexes program for 3D molecular space partitioning. http://iqc.udg.es/~eduard/ESI. Girona: Institute of Computational Chemistry and Catalysis
Keith A (2014) AIMall (v. 14.11.23). Overland Park: TK Gristmill Software (http://www.tkgristmill.com)
Portella G, Poater J, Solà M (2005) Assessment of the Clar’s aromatic pi-sextet rule by means of PDI, NICS, and HOMA indicators of local aromaticity. J Phys Org Chem 18:785–791
Zubarev DY, Boldyrev AI (2008) Revealing intuitively assessable chemical bonding patterns in organic aromatic molecules via adaptive natural density partitioning. J Org Chem 73:9251–9258
Popov IA, Boldyrev AI (2012) Chemical bonding in coronene, isocoronene, and circumcoronene. Eur J Org Chem 2012:3485–3491
Kabuto C, Oda M (1980) Crystal and molecular structure of 9,10-diphenylbicyclo[6.2.0]decapentaene a 10 π aromatic compound. Tetrahedron Lett 21:103–106
Papadakis R, Ottosson H (2015) The excited state antiaromatic benzene ring: a molecular Mr Hyde? Chem Soc Rev 44:6472–6493
Baird NC (1972) Quantum organic photochemistry. II: resonance and aromaticity in the lowest 3.pi.pi.* state of cyclic hydrocarbons. J Am Chem Soc 94:4941–4948
Roberts JD, Streitwieser A, Regan CM (1952) Small-ring compounds. X: molecular orbital calculations of properties of some small-ring hydrocarbons and free radicals1. J Am Chem Soc 74:4579–4582
Platt JR (1949) Classification of spectra of cata-condensed hydrocarbons. J Chem Phys 17:484–495
Soncini A, Havenith RWA, Fowler PW, Jenneskens LW, Steiner E (2002) Control of the diatropic pi ring current in strained benzenes: effects of annelation with cyclopropa, cyclobuta, and cyclobutadieno clampling groups. J Org Chem 67:4753–4758
Frank NL, Baldridge KK, Siegel JS (1995) Synthesis and characterization of trisbicyclo[2.1.1]hexabenzene, a highly strained bicycloannelated benzene. J Am Chem Soc 117:2102–2103
Fowler PW, Havenith RWA, Jenneskens LW, Soncini A, Steiner E (2001) Survival and extinction of delocalised ring currents in clamped benzenes. Chem Commun (22):2386–2387
Grant Hill J, Karadakov PB, Cooper DL (2006) The spin-coupled picture of clamped benzenes. Mol Phys 104:677–680
Feixas F, Matito E, Poater J, Solà M (2007) Aromaticity of distorted benzene rings: exploring the validity of different indicators of aromaticity. J Phys Chem A 111:4513–4521
Feixas F, Matito E, Poater J, Solà M (2008) On the performance of some aromaticity indices: a critical assessment using a test set. J Comput Chem 29:1543–1554
Solà M, Feixas F, Jiménez-Halla JOC, Matito E, Poater J (2010) A critical assessment of the performance of magnetic and electronic indices of aromaticity. Symmetry 2:1156–1179
Poater J, Visser R, Solà M, Bickelhaupt FM (2007) Polycyclic benzenoids: why kinked is more stable than straight. J Org Chem 72:1134–1142
Poater J, Bickelhaupt FM, Solà M (2007) Didehydrophenanthrenes: structure, singlet-triplet splitting, and aromaticity. J Phys Chem A 111:5063–5070
Dewar MJS, Li W-K (1974) MINDO [modified intermediate neglect of differential overlap]/3 study of the bisdehydrobenzenes. J Am Chem Soc 96:5569–5571
Feixas F, Vandenbussche J, Bultinck P, Matito E, Solà M (2011) Electron delocalization and aromaticity in low-lying excited states of archetypal organic compounds. Phys Chem Chem Phys 13:20690–20703
Rosenberg M, Dahlstrand C, Kilså K, Ottosson H (2014) Excited state aromaticity and antiaromaticity: opportunities for photophysical and photochemical rationalizations. Chem Rev 114:5379–5425
Acknowledgments
This work has been supported by the Ministerio de Economía y Competitividad (MINECO) of Spain (Project CTQ2014-54306-P) and the Generalitat de Catalunya (Project 2014SGR931, Xarxa de Referència en Química Teòrica i Computacional, ICREA Academia 2014 prize for M.S., and Grant No. 2014FI_B 00429 to O.E.B.). The EU under the FEDER Grant UNGI10-4E-801 (European Fund for Regional Development) has also funded this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to Prof. Dr. Alberto Vela as a proof of our admiration for his brilliant contributions to chemistry.
Published as part of the special collection of articles “Festschrift in honour of A. Vela”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Bakouri, O., Poater, J., Feixas, F. et al. Exploring the validity of the Glidewell–Lloyd extension of Clar’s π-sextet rule: assessment from polycyclic conjugated hydrocarbons. Theor Chem Acc 135, 205 (2016). https://doi.org/10.1007/s00214-016-1970-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1970-1