Abstract
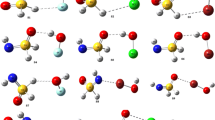
The interaction of hydrogen sulfide (H2S) with F, Cl, Br, and OH is investigated using ab initio methods to identify the two-center three-electron hemibond responsible for their complexation. The binding energies are found to be stronger than those in the analogous water complexes, but follow the same trend of increasing strength: F > Cl > Br > OH. The radicals are located nearly perpendicular to the H2S plane forming an angle of about 90°. Analysis of molecular orbitals and natural bond orbitals are carried out to understand the energetics, structures, and bonding characteristics of these hemibonded complexes.




Similar content being viewed by others
References
Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) Nature 326:655
Warneck P (2000) Chemistry of the natural atmosphere. Academic Press, San Diego
Glassman I (1996) Combustion, 3rd edn. Academic Press, San Diego
Li J, Dawes R, Guo H (2012) J Chem Phys 137:094304
Li J, Jiang B, Guo H (2013) J Chem Phys 138:074309
Li J, Jiang B, Guo H (2013) J Am Chem Soc 135:982
Nguyen TL, Li J, Dawes R, Stanton JF, Guo H (2013) J Phys Chem A 117:8864
Li J, Guo H (2013) Chin J Chem Phys 26:627
Li J, Dawes R, Guo H (2013) J Chem Phys 139:074302
Deskevich MP, Nesbitt DJ, Werner H-J (2004) J Chem Phys 120:7281
Li G, Zhou L, Li Q-S, Xie Y, Schaefer HF III (2012) Phys Chem Chem Phys 14:10891
Guo Y, Zhang M, Xie Y, Schaefer HF III (2013) J Chem Phys 139:041101
de Oliveira-Filho AGS, Ornellas FR, Bowman JM (2014) J Phys Chem Lett 5:706
Li J, Jiang B, Guo H (2013) Chem Sci 4:629
Li J, Li Y, Guo H (2013) J Chem Phys 138:141102
Pauling L (1931) J Am Chem Soc 53:3225
Gill PMW, Radom L (1988) J Am Chem Soc 110:4931
Fourré I, Silvi B (2007) Heteroatom Chem 18:135
Deng Y, Illies AJ, James MA, McKee ML, Peschke M (1995) J Am Chem Soc 117:420
McKee ML, Nicolaides A, Radom L (1996) J Am Chem Soc 118:10571
Nichols LS, Illies AJ (1999) J Am Chem Soc 121:9176
Braïda B, Hazebroucq S, Hiberty PC (2002) J Am Chem Soc 124:2371
Gao Y, Alecu IM, Hsieh P-C, Morgan BP, Marshall P, Krasnoperov LN (2006) J Phys Chem A 110:6844
Aloisio S (2006) Chem Phys 326:335
Joshi R, Ghanty TK, Naumov S, Mukherjee T (2007) J Phys Chem A 111:2362
Pathak AK, Mukherjee T, Maity DK (2008) J Mol Struct Theochem 851:158
Fourré I, Bergès J, Houée-Levin C (2010) J Phys Chem A 114:7359
Monge-Palacios M, Espinosa-Garcia J (2010) J Phys Chem A 114:4418
Chipman DM (2011) J Phys Chem A 115:1161
Yamaguchi M (2011) J Phys Chem A 115:14620
Codorniu-Hernandez E, Boese AD, Kusalik PG (2013) Can J Chem 91:544
Adler TB, Knizia G, Werner H-J (2007) J Chem Phys 127:221106
Knizia G, Adler TB, Werner H-J (2009) J Chem Phys 130:054104
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96:6796
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M et al (2012) MOLPRO, version 2012.1, a package of ab initio programs. http://www.molpro.net
Lynch BJ, Fast PL, Harris M, Truhlar DG (2000) J Phys Chem A 104:4811
Weinhold F, Landis CR (2005) Valency and bonding. A natural bond orbital donor–acceptor perspective. Cambridge University Press, Cambridge
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, J. A., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Vol. A.01, Wallingford CT
Tentscher PR, Arey JS (2013) J Chem Theo Comput 9:1568
Hayes WM (2014) CRC handbook of chemistry and physics. Taylor and Francis, Boca Raton
Acknowledgments
This work was supported by the Department of Energy (DE-FG02-05ER15694). The computations were performed at the National Energy Research Scientific Computing (NERSC) Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Guosen Yan and published as part of the special collection of articles celebrating his 85th birthday.
Rights and permissions
About this article
Cite this article
Alday, B., Johnson, R., Li, J. et al. Hemibond complexes between H2S and free radicals (F, Cl, Br, and OH). Theor Chem Acc 133, 1540 (2014). https://doi.org/10.1007/s00214-014-1540-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1540-3