Abstract
Rationale
Extracellular proteolytic activity plays an important role in memory formation and the preservation of cognitive function. Previous studies have shown increased levels of plasminogen activator inhibitor-1 (PAI-1) in the brain of mouse models of Alzheimer’s disease (AD) and plasma of AD patients, associated with memory and cognitive decline; however, the exact function of PAI-1 in AD onset and progression is largely unclear.
Objective
In this study, we evaluated a novel PAI-1 inhibitor, TM5A15, on its ability to prevent or reverse memory deficits and decrease Aβ levels and plaque deposition in APP/PS1 mice.
Methods
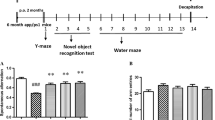
We administered TM5A15 mixed in a chow diet to 3-month and 9-month-old APP/PS1 mice before and after neuropathological changes were distinguishable. We then evaluated the effects of TM5A15 on memory function and neuropathology at 9 months and 18 months of age.
Results
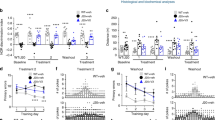
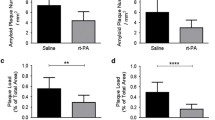
In the younger mice, 6 months of TM5A15 treatment protected against recognition and short-term working memory impairment. TM5A15 also decreased oligomer levels and amyloid plaques, and increased mBDNF expression in APP/PS1 mice at 9 months of age. In aged mice, 9 months of TM5A15 treatment did not significantly improve memory function nor decrease amyloid plaques. However, TM5A15 treatment showed a trend in decreasing oligomer levels in APP/PS1 mice at 18 months of age.
Conclusion
Our results suggest that PAI-1 inhibition could improve memory function and reduce the accumulation of amyloid levels in APP/PS1 mice. Such effects are more prominent when TM5A15 is administered before advanced AD pathology and memory deficits occur.








Similar content being viewed by others
References
Akhter H, Huang WT, van Groen T et al (2018) A Small molecule inhibitor of plasminogen activator inhibitor-1 reduces brain amyloid-beta load and improves memory in an animal model of Alzheimer’s disease. J Alzheimers Dis 64(2):447–457
Barker R, Kehoe PG, Love S (2012) Activators and inhibitors of the plasminogen system in Alzheimer’s disease. J Cell Mol Med 16(4):865–876
Bi OS, Suh N, Kim I et al (2015) Impacts of aging and amyloid-beta deposition on plasminogen activators and plasminogen activator inhibitor-1 in the Tg2576 mouse model of Alzheimer’s disease. Brain Res 1597:159–167
Bloom GS (2014) Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71(4):505–508
Busche MA, Hyman BT (2020) Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat Neurosci 23(10):1183–1193
Cline EN, Das A, Bicca MA et al (2019) A novel crosslinking protocol stabilizes amyloid beta oligomers capable of inducing Alzheimer’s-associated pathologies. J Neurochem 148(6):822–836
Diaz A, Merino P, Guo JD et al (2020) Urokinase-type plasminogen activator protects cerebral cortical neurons from soluble abeta-induced synaptic damage. J Neurosci 40(21):4251–4263
Eren M, Boe AE, Klyachko EA et al (2014) Role of plasminogen activator inhibitor-1 in senescence and aging. Semin Thromb Hemost 40(6):645–651
Eren M, Place AT, Thomas PM et al (2017) PAI-1 is a critical regulator of FGF23 homeostasis. Sci Adv 3(9):e1603259
Fabbro S, Seeds NW (2009) Plasminogen activator activity is inhibited while neuroserpin is up-regulated in the Alzheimer disease brain. J Neurochem 109(2):303–315
Fekih-Mrissa N, Mansour M, Sayeh A et al (2017) The plasminogen activator inhibitor 1 4G/5G Polymorphism and the risk of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 32(6):342–346
Gerenu G, Martisova E, Ferrero H et al (2017) Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits. Biochim Biophys Acta Mol Basis Dis 4:991–1001
Grabher BJ (2018) Effects of Alzheimer disease on patients and their family. J Nucl Med Technol 46(4):335–340
Hardigan T, Ward R, Ergul A (2016) Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clin Sci (Lond) 130(20):1807–1822
Hendrickx JO, De Moudt S, Calus E et al (2022) Age-related cognitive decline in spatial learning and memory of C57BL/6J mice. Behav Brain Res 418:113649
Huang LK, Chao SP, Hu CJ (2020) Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 27(1):18
Jack CR Jr, Knopman DS, Jagust WJ et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12(2):207–216
Jacobsen JS, Comery TA, Martone RL et al (2008) Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci U S A 105(25):8754–8759
Jiang CS, Rana T, Jin LW et al (2023) Aging, plasminogen activator inhibitor 1, brain cell senescence, and Alzheimer’s disease. Aging Dis 14(2):515–528
Kupershmidt L, Youdim MBH (2023) The Neuroprotective activities of the novel multi-target iron-chelators in models of Alzheimer’s disease, amyotrophic lateral sclerosis and aging. Cells 12(5):763
Kutz SM, Higgins CE, Higgins PJ (2012) Novel combinatorial therapeutic targeting of PAI-1 (SERPINE1) gene expression in Alzheimer’s disease. Mol Med Ther 1(2):106
Lambert MP, Velasco PT, Chang L et al (2007) Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem 100(1):23–35
Lee SH, Eren M, Vaughan DE et al (2012) A plasminogen activator inhibitor-1 inhibitor reduces airway remodeling in a murine model of chronic asthma. Am J Respir Cell Mol Biol 46(6):842–846
Lee TW, Tsang VW, Loef EJ et al (2017) Physiological and pathological functions of neuroserpin: Regulation of cellular responses through multiple mechanisms. Semin Cell Dev Biol 62:152–159
Leger M, Quiedeville A, Bouet V et al (2013) Object recognition test in mice. Nat Protoc 8(12):2531–2537
Li X, Zhang H, Yang L et al (2023) Inhibition of NLRP1 inflammasome improves autophagy dysfunction and Abeta disposition in APP/PS1 mice. Behav Brain Funct 19(1):7
Liu RM (2022) Aging, cellular senescence, and Alzheimer’s disease. Int J Mol Sci 23(4). https://doi.org/10.3390/ijms23041989
Liu RM, van Groen T, Katre A et al (2011) Knockout of plasminogen activator inhibitor 1 gene reduces amyloid beta peptide burden in a mouse model of Alzheimer’s disease. Neurobiol Aging 32(6):1079–1089
Locci A, Orellana H, Rodriguez G et al (2021) Comparison of memory, affective behavior, and neuropathology in APP(NLGF) knock-in mice to 5xFAD and APP/PS1 mice. Behav Brain Res 404:113192
Lu B, Nagappan G, Guan X et al (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14(6):401–416
Lueptow LM (2017) Novel Object recognition test for the investigation of learning and memory in mice. J Vis Exp Epub ahead of print 20170830:–126. https://doi.org/10.3791/55718
Mantile F, Prisco A (2020) Vaccination against beta-amyloid as a strategy for the prevention of Alzheimer’s disease. Biology (Basel) 9(12):425
McClarty B, Rodriguez G, Dong H (2021) Dose effects of histone deacetylase inhibitor tacedinaline (CI-994) on antipsychotic haloperidol-induced motor and memory side effects in aged mice. Front Neurosci 15:674745
Melchor JP, Pawlak R, Strickland S (2003) The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci 23(26):8867–8871
Montalvo-Ortiz JL, Fisher DW, Rodriguez G et al (2017) Histone deacetylase inhibitors reverse age-related increases in side effects of haloperidol in mice. Psychopharmacology (Berl) 234(16):2385–2398
Mori T, Koyama N, Yokoo T et al (2020) Gallic acid is a dual alpha/beta-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J Biol Chem 295(48):16251–16266
Mou X, Peterson CB, Prosser RA (2009) Tissue-type plasminogen activator-plasmin-BDNF modulate glutamate-induced phase-shifts of the mouse suprachiasmatic circadian clock in vitro. Eur J Neurosci 30(8):1451–1460
NIA (2023) Alzheimer’s Disease Fact Sheet. Available at: https://www.nia.nih.gov/health/alzheimers-disease-factsheet#:~:text=Alzheimer%E2%80%99s%20is%20currently%20ranked%20asof%20dementia%20among%20older%20adults.
O’Leary TP, Brown RE (2013) Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn Mem 20(2):85–96
Oh J, Lee HJ, Song JH et al (2014a) Plasminogen activator inhibitor-1 as an early potential diagnostic marker for Alzheimer’s disease. Exp Gerontol 60:87–91
Oh SB, Byun CJ, Yun JH et al (2014b) Tissue plasminogen activator arrests Alzheimer’s disease pathogenesis. Neurobiol Aging 35(3):511–519
Park L, Zhou J, Koizumi K et al (2020) tPA deficiency underlies neurovascular coupling dysfunction by amyloid-beta. J Neurosci 40(42):8160–8173
Pentz R, Iulita MF, Ducatenzeiler A et al (2021) The human brain NGF metabolic pathway is impaired in the preclinical and clinical continuum of Alzheimers disease. Mol Psychiatry 26(10):6023–6037
Reutzel M, Grewal R, Dilberger B et al (2020) Cerebral Mitochondrial function and cognitive performance during aging: a longitudinal study in NMRI Mice. Oxid Med Cell Longev 2020:4060769
Schindowski K, Belarbi K, Buee L (2008) Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav 7(Suppl 1):43–56
Sillen M, Declerck PJ (2021) A narrative review on plasminogen activator inhibitor-1 and its (patho)physiological role: to target or not to target? Int J Mol Sci 22(5):2721
Simon DI, Simon NM (2013) Plasminogen activator inhibitor-1: a novel therapeutic target for hypertension? Circulation 128(21):2286–2288
Suidan GL, Singh PK, Patel-Hett S et al (2018) Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv 2(9):954–963
Sun T, Ghosh AK, Eren M et al (2019) PAI-1 contributes to homocysteine-induced cellular senescence. Cell Signal 64:109394
Sutton R, Keohane ME, VanderBerg SR et al (1994) Plasminogen activator inhibitor-1 in the cerebrospinal fluid as an index of neurological disease. Blood Coagul Fibrinolysis 5(2):167–171
Szabo A, Farkas S, Fazekas C et al (2023) Temporal appearance of enhanced innate anxiety in Alzheimer model mice. Biomedicines. 2:262. https://doi.org/10.3390/biomedicines11020262
Tang MY, Gorin FA, Lein PJ (2022) Review of evidence implicating the plasminogen activator system in blood-brain barrier dysfunction associated with Alzheimer’s disease. Ageing Neurodegener Dis 2:2
Tucker HM, Kihiko M, Caldwell JN et al (2000) The plasmin system is induced by and degrades amyloid-beta aggregates. J Neurosci 20(11):3937–3946
Vaughan DE, Rai R, Khan SS et al (2017) Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler Thromb Vasc Biol 37(8):1446–1452
Viana TG, Almeida-Santos AF, Aguiar DC et al (2013) Effects of aripiprazole, an atypical antipsychotic, on the motor alterations induced by acute ethanol administration in mice. Basic Clin Pharmacol Toxicol 112(5):319–324
Wang DS (2006) Dickson DW and Malter JS (2006) beta-Amyloid degradation and Alzheimer’s disease. J Biomed Biotechnol 3:58406
Wang J, Yuan Y, Cai R et al (2018) Association between plasma levels of PAI-1, tPA/PAI-1 molar ratio, and mild cognitive impairment in chinese patients with type 2 diabetes mellitus. J Alzheimers Dis 63(2):835–845
Yarmolinsky J, Bordin Barbieri N, Weinmann T et al (2016) Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep 6:17714
Yepes M (2021) The plasminogen activating system in the pathogenesis of Alzheimer’s disease. Neural Regen Res 16(10):1973–1977
Ziliotto N, Bernardi F, Piazza F (2021) Hemostasis components in cerebral amyloid angiopathy and Alzheimer’s disease. Neurol Sci 42(8):3177–3188
Acknowledgements
This study was supported by the National Institution of Aging (1R01AG062249, Dong) and the National Heart, Lung, and Blood Institute (R01HL051387, Vaughan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
S. Figure 1. TM5A15 intake and body weight in wild-type and APP/PS1 mice. A. The body weight of mice measured before and after 6 months of the vehicle or TM5A15 treatment. Mice showed no significant change in body weight between groups. B. Food intake of vehicle and TM5A15 was measured weekly. Weekly measurements showed no difference in food intake between groups that were treated for 6 months. C. The body weight of mice was measured before and after 9 months of the vehicle or TM5A15 treatment. Mice showed no significant change in body weight between groups. D. Food intake of the vehicle and TM5A15 was measured weekly. Weekly measurements showed no difference in food intake between groups that were treated for 9 months. (PDF 106 kb)
ESM 2
S. Figure 2. PAI-1 activity levels on WT and APP/PS1 after completion of the vehicle and TM5A15 treatment. Data are presented as Mean±SEM, n=14, * p= 0.0361. (PDF 36 kb)
ESM 3
S. Figure 3. Effect of vehicle treatment on PAI-1 signaling and downstream factors in 9 month and 18 month-old WT and APP/PS1 mice. Protein quantification by western blot was conducted, and t-PA, (A: * p= 0.0117, ** p= 0.0090) and PAI-1 (B: * p= 0.0231, WT 9mo vs WT 18mo ** p=0.0013, APP/PS1 9mo vs APP/PS1 18 mo ** p= 0.0042, ***p = 0.0003) in untreated WT and APP/PS1 groups were determined. Data are presented as Mean±SEM, n=5-7. (PDF 86 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez, G., Eren, M., Haupfear, I. et al. Pharmacological inhibition of plasminogen activator inhibitor-1 prevents memory deficits and reduces neuropathology in APP/PS1 mice. Psychopharmacology 240, 2641–2655 (2023). https://doi.org/10.1007/s00213-023-06459-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06459-8