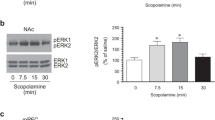
Abstract
Muscarinic acetylcholine receptors (mAChRs) have been shown to play significant roles in the regulation of normal cognitive processes in the hippocampus, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) are also involved in these processes. This study aims to explore the mAChR-mediated regulation of AMPARs GluA2 trafficking and to reveal the key proteins and the signaling cascade involved in this process. Primary hippocampal neurons, as cell models, were treated with agonist 77-LH-28-1 and antagonist VU0255035, Fsc231, and APV. C57BL/6J male mice were stereotactically injected with 77-LH-28-1 and Fsc231 to obtain hippocampal slices. The trafficking of GluA2 was detected by surface biotinylation and immunostaining. Activation of M1 mAChRs promoted endocytosis and decreased the postsynaptic localization of the AMPA receptor subunit GluA2 and that phosphorylation of GluA2 at Ser880 was increased by M1 mAChR activity. Fsc231 blocked the endocytosis and postsynaptic localization of GluA2 induced by 77-LH-28-1 without affecting the phosphorylation of Ser880. PICK1 was required for M1 mAChR-mediated GluA2 endocytosis and downstream of phosphorylation of GluA2-Ser880, and the PICK1-GluA2 interaction was essential for M1 mAChR-mediated postsynaptic expression of GluA2. Taken together, our results show a functional correlation of M1 mAChRs with GluA2 and the role of PICK1 in their interplay.
Graphical abstract

The schematic diagram for the modulation of GluA2 trafficking by M1 mAChRs. Activation of M1 mAChRs induces PKC activation, and the interaction of PICK1-GluA2 determines the endocytosis and postsynaptic localization of GluA2




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the present study are not publicly available due to unfinished follow-up research but are available from the corresponding author upon reasonable request.
References
Bubser M, Byun N, Wood MR, Jones CK (2012) Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb Exp Pharmacol:121–166
Cheng H, Li J, Fazlieva R, Dai Z, Bu Z, Roder H (2009) Autoinhibitory interactions between the PDZ2 and C-terminal domains in the scaffolding protein NHERF1. Structure 17:660–669
Daw MI, Chittajallu R, Bortolotto ZA et al (2000) PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28:873–886
Diering GH, Huganir RL (2018) The AMPA receptor code of synaptic plasticity. Neuron 100:314–329
Doucet MV, Levine H, Dev KK, Harkin A (2013) Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology 38:1575–1584
Ehlers MD (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28:511–525
Ennion S, Hagan S, Evans RJ (2000) The role of positively charged amino acids in ATP recognition by human P2X(1) receptors. J Biol Chem 275:29361–29367
Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W (2008) Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci 28:1469–1478
Freudenberg F, Celikel T, Reif A (2015) The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (ampa) receptors in depression: central mediators of pathophysiology and antidepressant activity. Neurosci Biobehav Rev 52:193–206
Hasselmann H (2014) Scopolamine and depression: a role for muscarinic antagonism. CNS Neurol Disord Drug Targets 13:673–683
Hu XD, Huang Q, Yang X, Xia H (2007) Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J Neurosci 27:4674–4686
Huganir RL, Nicoll RA (2013) AMPARs and synaptic plasticity: the last 25 years. Neuron 80:704–717
Janowsky DS (2011) Serendipity strikes again: scopolamine as an antidepressant agent in bipolar depressed patients. Curr Psychiatry Rep 13:443–445
Jiang H, Liu JP, Xi K, Liu LY, Kong LY, Cai J, Cai SQ, Han XY, Song JG, Yang XM, Wan Y, Xing GG (2021) Contribution of AMPA receptor-mediated LTD in LA/BLA-CeA pathway to comorbid aversive and depressive symptoms in neuropathic pain. J Neurosci 41:7278–7299
Jin W, Ge WP, Xu J et al (2006) Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci 26:2380–2390
Kam AY, Liao D, Loh HH, Law PY (2010) Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci 30:15304–15316
Kim CH, Chung HJ, Lee HK, Huganir RL (2001) Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A 98:11725–11730
Langmead CJ, Austin NE, Branch CL, Brown JT, Buchanan KA, Davies CH et al (2008) Characterization of a CNS penetrant, selective m1 muscarinic receptor agonist, 77-lh-28-1. Br J Pharmacol 154:1104–1115
Liu SJ, Zukin RS (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134
Lu W, Shi Y, Jackson AC et al (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62:254–268
Madsen KL, Thorsen TS, Rahbek-Clemmensen T, Eriksen J, Gether U (2012) Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway. J Biol Chem 287:12293–12308
Martin AE, Schober DA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE et al (2017) Further evaluation of mechanisms associated with the antidepressantlike signature of scopolamine in mice. CNS Neurol Disord Drug Targets 16:492–500
Matsuda S, Launey T, Mikawa S, Hirai H (2000) Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J 19:2765–2774
Opazo P, Sainlos M, Choquet D (2012) Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol 22:453–460
Osten P, Khatri L, Perez JL et al (2000) Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron 27:313–325
Parkinson GT, Chamberlain S, Jaafari N, Turvey M, Mellor JR, Hanley JG (2018) Cortactin regulates endo-lysosomal sorting of AMPARs via direct interaction with GluA2 subunit. Sci Rep 8:4155
Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB (2001) PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 21:5417–5428
Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109
Plant K, Pelkey KA, Bortolotto ZA et al (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604
Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev P, Martin D et al (2013) Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology 38:2101–2108
Purkey AM, Dell'Acqua ML (2020) Phosphorylation-dependent regulation of Ca(2+)-permeable AMPA receptors during hippocampal synaptic plasticity. Front Synaptic Neurosci 12:8
Scarr E (2012) Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci Ther 18:369–379
Scheiderer CL, McCutchen E, Thacker EE et al (2006) Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3-CA1 synapses. J Neurosci 26:3745–3756
Seidenman KJ, Steinberg JP, Huganir R, Malinow R (2003) Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 23:9220–9228
Sheng M, Sala C (2001) PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24:1–29
Siafis S, Papazisis G (2018) Detecting a potential safety signal of antidepressants and type 2 diabetes: a pharmacovigilance-pharmacodynamic study. Br J Clin Pharmacol 84:2405–2414
Steinberg JP, Takamiya K, Shen Y et al (2006) Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49:845–860
Terashima A, Pelkey KA, Rah JC et al (2008) An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 57:872–882
Thorsen TS, Madsen KL, Rebola N et al (2010) Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc Natl Acad Sci U S A 107:413–418
Traynelis SF, Wollmuth LP, McBain CJ et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496
Volk L, Kim CH, Takamiya K, Yu Y, Huganir RL (2010) Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc Natl Acad Sci U S A 107:21784–21789
Wang WL, Yeh SF, Chang YI et al (2003) PICK1, an anchoring protein that specifically targets protein kinase Calpha to mitochondria selectively upon serum stimulation in NIH 3T3 cells. J Biol Chem 278:37705–37712
Weaver CD, Sheffler DJ, Lewis LM, Bridges TM, Williams R, Nalywajko NT et al (2009) Discovery and development of a potent and highly selective small molecule muscarinic acetylcholine receptor subtype I (machR 1 or m1) antagonist in vitro and in vivo probe. Curr Top Med Chem 9:1217–1226
Witkin JM, Smith JL, Golani LK, Brooks EA, Martin AE (2020) Involvement of muscarinic receptor mechanisms in antidepressant drug action. Adv Pharmacol 89:311–356
Yao Y, Kelly MT, Sajikumar S et al (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 28:7820–7827
Zeng M, Chen X, Guan D et al (2018) Reconstituted postsynaptic density as a molecular platform for understanding synapse formation and plasticity. Cell 174:1172–1187.e16
Zhou Z, Liu A, Xia S et al (2018) The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat Neurosci 21:50–62
Acknowledgements
We are very grateful to Professor Yu Qiu for providing us with 77-LH-28-1 and to Dr. Lanxue Zhao for her methodological knowledge.
Funding
This work was supported by the National Natural Science Foundation of China (81703478).
Author information
Authors and Affiliations
Contributions
Y.-H. Yan designed the study and supervised the work; Z.-Y. Zhu, W.-J. Wang, and C. Gu performed the experiment; M. Wang and Y.-H. Yan analyzed the data; Z.-Y. Zhu and Y.-H. Yan wrote the manuscript; and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Children’s Hospital of Soochow University (March 2018).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 358 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Z., Wang, W., Gu, C. et al. The M1 muscarinic acetylcholine receptor regulates the surface expression of the AMPA receptor subunit GluA2 via PICK1. Psychopharmacology 240, 239–248 (2023). https://doi.org/10.1007/s00213-022-06304-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06304-4