Abstract
Rationale
Nicotine use disorder can alter synaptic plasticity correlated with learning and memory process. G protein–coupled receptor 55 (GPR55), a novel cannabinoid receptor, which is highly expressed in the central nervous system, plays a prominent role in learning and memory. However, the role of GPR55 in nicotine use disorder remains unclear.
Methods
In this study, we used the conditioned place preference (CPP) paradigm, a standard and well-established model for evaluating the rewarding effect of drug abuse, to investigate nicotine use disorder behavior in mice. After behavioral tests, the effect of GPR55 on nicotine response was evaluated using Western blotting, immunofluorescence staining, whole-cell patch-clamp recordings, and ELISA.
Results
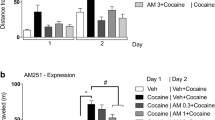
GPR55 activation significantly reduced nicotine-CPP behavior by decreasing the spontaneous excitatory postsynaptic currents frequency in the nucleus accumbens (NAc) and the release of dopamine in serum. Furthermore, we found that the inhibition effects of nicotine response were mediated by phosphorylation of AMPAR. The PI3K-Akt signaling was involved in nicotine-CPP via GPR55 activation.
Conclusion
Our findings showed that GPR55 in the NAc plays a specific role in blocking nicotine-CPP behavior and might be a potential target for the treatment of nicotine use disorder.







Similar content being viewed by others
References
Alavi MS, Hosseinzadeh H, Shamsizadeh A, Roohbakhsh A (2016) The effect of O-1602, an atypical cannabinoid, on morphine-induced conditioned place preference and physical dependence. Pharmacological Reports : PR 68:592–597
Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD (2016) Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatr 6: e713
Benowitz NL (2010) Nicotine addiction. N Engl J Med 362:2295–2303
Briand LA, Deutschmann AU, Ellis AS, Fosnocht AQ (2016) Disrupting GluA2 phosphorylation potentiates reinstatement of cocaine seeking. Neuropharmacology 111:231–241
Collaborators GBDT (2017) Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389:1885–1906
Collo G, Bono F, Cavalleri L, Plebani L, Mitola S, Merlo Pich E, Millan MJ, Zoli M, Maskos U, Spano P, Missale C (2013) Nicotine-induced structural plasticity in mesencephalic dopaminergic neurons is mediated by dopamine D3 receptors and Akt-mTORC1 signaling. Mol Pharmacol 83:1176–1189
David V, Besson M, Changeux JP, Granon S, Cazala P (2006) Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 50:1030–1040
Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA 110:9124–9129
Good CH, Lupica CR (2010) Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci: the Off J Soc Neurosci 30:7900–7909
Grabus SD, Martin BR, Brown SE, Damaj MI (2006) Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology 184:456–463
Hall BJ, Slade S, Wells C, Rose JE, Levin ED (2015) Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacol Biochem Behav 130:84–89
Han J, Liu Z, Ren W, Zhang X (2011) Counteractive effects of cannabinoid and nicotine-addictive behavior. NeuroReport 22:181–184
Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287:2262–2267
Hughes JR, Keely J, Naud S (2004) Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 99:29–38
Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61:340–350
Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT (2012) Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci 15:1556–1562
Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K (2008) GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA 105:2699–2704
Le Foll B, Goldberg SR (2006) Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology 184:367–381
Liu SB, Zhang N, Guo YY, Zhao R, Shi TY, Feng SF, Wang SQ, Yang Q, Li XQ, Wu YM, Ma L, Hou Y, Xiong LZ, Zhang W, Zhao MG (2012) G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci: the Off J Soc Neurosci 32:4887–4900
Liu Z, Han J, Jia L, Maillet JC, Bai G, Xu L, Jia Z, Zheng Q, Zhang W, Monette R, Merali Z, Zhu Z, Wang W, Ren W, Zhang X (2010) Synaptic neurotransmission depression in ventral tegmental dopamine neurons and cannabinoid-associated addictive learning. PloS one 5: e15634.
Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK (2012) Glutamatergic synapse formation is promoted by alpha7-containing nicotinic acetylcholine receptors. J Neurosci: the Off J Soc Neurosci 32:7651–7661
Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL (1999) Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 24:389–399
Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232
Mameli M, Balland B, Lujan R, Luscher C (2007) Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317:530–533
Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357
Mao D, Gallagher K, McGehee DS (2011) Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci: the Off J Soc Neurosci 31:6710–6720
Marichal-Cancino BA, Sanchez-Fuentes A, Mendez-Diaz M, Ruiz-Contreras AE, Prospero-Garcia O (2016) Blockade of GPR55 in the dorsolateral striatum impairs performance of rats in a T-maze paradigm. Behav Pharmacol 27:393–396
Newton AC, Bootman MD, Scott JD (2016) Second Messengers. Cold Spring Harbor perspectives in biology 8.
Robertson-Gray OJ, Walsh SK, Ryberg E, Jonsson-Rylander AC, Lipina C, Wainwright CL (2019) l-alpha-Lysophosphatidylinositol (LPI) aggravates myocardial ischemia/reperfusion injury via a GPR55/ROCK-dependent pathway. Pharmacol Res Perspect 7: e00487.
Rogawski MA (2013) AMPA receptors as a molecular target in epilepsy therapy. Acta neurologica Scandinavica Supplementum: 9–18.
Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152:1092–1101
Shi QX, Yang LK, Shi WL, Wang L, Zhou SM, Guan SY, Zhao MG, Yang Q (2017) The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol Brain 10:38
Sun X, Zhao Y, Wolf ME (2005) Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci: the Off J Soc Neurosci 25:7342–7351
Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, Zhao MG, Zhuo M (2008) FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 59:634–647
Funding
This research was supported by the National Natural Science Foundation Grant No. 81870893 (to QY) and the National Postdoctoral Program for Innovative Talents No. BX20160025 (to QY).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Q., Yu, J., Li, X. et al. Cannabinoid receptor GPR55 activation blocks nicotine use disorder by regulation of AMPAR phosphorylation. Psychopharmacology 238, 3335–3346 (2021). https://doi.org/10.1007/s00213-021-05949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05949-x