Abstract
Rationale and objectives
The present study characterized the behavioral pharmacology of a novel, mixed-action delta-selective (78:1) opioid receptor agonist, BBI-11008. This glycopeptide drug candidate was tested in assays assessing antinociception (acute, inflammatory, and neuropathic pain-like conditions) and side-effect endpoints (respiratory depression and drug self-administration).
Results
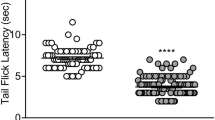
BBI-11008 had a 78-fold greater affinity for the delta opioid receptor than the mu receptor, and there was no binding to the kappa opioid receptor. BBI-11008 (3.2–100; 10–32 mg kg−1, i.v.) and morphine (1–10; 1–3.2 mg kg−1, i.v.) produced antinociceptive and anti-allodynic effects in assays of acute thermal nociception and complete Freund’s adjuvant (CFA)-induced inflammatory pain, with BBI-11008 being less potent than morphine in both assays. BBI-11008 (1–18 mg kg−1, i.v.) had similar efficacy to gabapentin (10–56 mg kg−1, i.v.) in a spinal nerve ligation (SNL) model of neuropathic pain. In the respiration assay, with increasing %CO2 exposure, BBI-11008 produced an initial increase (32 mg kg−1, s.c.) and then decrease (56 mg kg−1, s.c.) in minute volume (MV) whereas morphine (3.2–32 mg kg−1, s.c.) produced dose-dependent decreases in MV. In the drug self-administration procedure, BBI-11008 did not maintain self-administration at any dose tested.
Conclusions
These results suggest that the glycopeptide drug candidate possesses broad-spectrum antinociceptive and anti-allodynic activity across a range of pain-like conditions. Relative to morphine or fentanyl, the profile for BBI-11008 in the respiration and drug self-administration assays suggests that BBI-11008 may have less pronounced deleterious side effects. Continued assessment of this compound is warranted.









Similar content being viewed by others
References
Anand JP, Montgomery D (2018) Multifunctional opioid ligands. In: Jutkiewicz EM (ed) Delta opioid receptor pharmacology and therapeutic applications, Handbook of experimental pharmacology, vol 247. Springer, pp 21–52
Anand JP, Boyer BT, Mosberg HI, Jutkiewicz EM (2016) The behavioral effects of a mixed efficacy antinociceptive peptide, VRP26, following chronic administration in mice. Psychopharm 233:2479–2487
Antman EM (2017) Evaluating the cardiovascular safety of nonsteroidal anti-inflammatory drugs. Circul 135(21):2062–2072
Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F (1995) SNC80 a selective nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther 273:359–366
Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R (2000) Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem 43(13):2586–2590
Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR (2002) Comparison of receptor mechanism and efficacy requirements for δ-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther 303:723–729
Chaplan SR, Back FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22(23):3099–3108
Colburn RW, Rickman AJ, DeLeo JA (1999) The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 157:289–304
Craft RM (2007) Modulation of pain by estrogens. Pain 132:S3–S12
Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL (2001) Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesth 94(5):824–832
Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, Dhanasekaran M, Keyari CM, Yamamura HI, Porreca F, Hruby VJ, Polt R, Bilsky EJ (2004) Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharmacol Exp Ther 311:290–297
Erspamer V, Melchiorri P, Falconieir-Erspamer G et al (1989) Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci U S A 86(13):5188–5192
Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10(5):447–485
Fischer BD (2011) Preclinical assessment of drug combinations for the treatment of pain: isobolographic and dose-addition analysis of the opioidergic system. CNS Neurol Disord Drug Targets 10(5):529–535
Fishman SM, Condon J, Holtsman M (2004) Common opioid-related side effects. In: Warfield CA, Bajwa ZH (eds) Principles and practice of pain medicine, 3rd edn. McGraw-Hill, pp 612–615
Godfrey RG (1996) A guide to the understanding and use of tricyclic antidepressants in the overall management of fibromyalgia and other chronic pain syndromes. Arch Intern Med 156(10):1047–1052
Greenspan JD, Craft RM, LeResche L et al (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132:S26–S45
Heck SD, Faraci WS, Kelbaugh PR, Saccomano NA, Thadeio PF, Volkmann RA (1996) Posttranslational amino acid epimerization: enzyme-catalyzed isomerization of amino acid residues in peptide chains. Proc Natl Acad Sci U S A 93(9):4036–4039
Institute of Medicine (IOM) (2011) Relieving pain in America: a blueprint for transforming prevention, care, education, and research. The National Academies Press, Washington, DC
Janssen PA, Niemegeers CJ, Dony JG (1963). The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. Jun;13:502–7
Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH (2004) δ-Opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther 309:173–181
Kaye AD, Cornett EM, Helander E, Menard B, Hsu E, Hart B, Brunk A (2017) An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin 35(2):55–71
Krashin D, Murinova N, Jumelle P, Ballantyne J (2015) Opioid risk assessment in palliative medicine. Expert Opin Drug Saf 14(7):1023–1033
Kreil G, Barra D, Simmaco M et al (1989) Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for delta opioid receptors. Eur J Pharmacol 162(1):123–128
Lei W, Vekariya RH, Subramaniam A, Streicher JM (2019) A novel mu-delta opioid agonist demonstrates enhanced efficacy with reduced tolerance and dependence in mouse neuropathic pain models. J Pain pii: S1526-5900(19)30745-X. https://doi.org/10.1016/j.jpain.2019.05.017
Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R (2012) Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Future Med Chem 4(2):205–226
Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM (2003) Presynaptic delta opioid receptors differentially modulate rhythm and pattern generation in the ventral respiratory group of the rat. Neurosci 121(4):959–973
Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ (2011) In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice. J Pharmacol Exp Ther 336(3):767–778
Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL (2010) Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med 60(3):177–188
Melchiorri P, Negri L (1996) The dermorphin peptide family. Gen Pharmacol 27(7):1099–1107
Mello NK, Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424
Morin-Surun MP, Boudinot E, Gacel G, Champagnat J, Roques BP, Denavit-Saubie M (1984) Different effects of mu and delta opiate agonists on respiration. Eur J Pharmacol 98(2):235–240
Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM (2014) Development of a bioavailable μ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J Med Chem 57:3148–3153
Negus SS (2019) Core outcome measures in preclinical assessment of candidate analgesics. Pharmacol Rev 71:225–266
Negus SS, Vanderah TW, Brandt MR, Bilsky ER, Becerra L, Borsook D (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future directions. J Pharmacol Exp Ther 319:507–514
Pazos A, Florez J (1984) A comparative study in rats of the respiratory depression and analgesia induced by mu- and delta-opioid agonists. Eur J Pharmacol 99(1):15–21
Schiller PW, Weltrowska G, Schmidt R, Nguyen TMD, Berezowska I, Lemieux C, Chunb NN, Carpenter KA, Wilkes BC (1995) Four different kinds of opioid peptides with mixed μ agonist/δ antagonist properties. Analgesia 1(4–6):703–706
Stevenson GW, Bilsky EJ, Negus SS (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7(6):408–416
Stevenson GW, Luginbuhl A, Dunbar C, LaVigne J, Dutra J, Atherton P, Bell B, Cone K, Giuvelis D, Polt R, Streicher JM, Bilsky EJ (2015) The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol Biochem Behav 132:49–55
Su YF, McNutt RW, Chang KJ (1998) Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther 287(3):815–823
Suzuki T, Tsuji M, Mori T, Misawa M, Endoh T, Nagase H (1995) Effects of a highly selective nonpeptide δ opioid receptor agonist, TAN-67, on morphine-induced antinociception in mice. Life Sci 57(2):155–168
Taurog JD, ARgentieri DC, McReynolds RA (1988) Adjuvant arthritis. Methods Enzymol 162:339–355
Taylor CP (2009) Mechanisms of analgesia by gabapentin and pregabalin—calcium channel α2-δ [Cavα2-δ] ligands. Pain 142:13–16
Thomsen M, Caine SB (2005) Chronic intravenous drug self-administration in rodents. Curr Protoc Neurosci Suppl 9(20):1–9.20.40
Wojciechowski P, Szereda-Przestaszewska M, Lipkowski AW (2011) Delta opioid receptors contribute to the cardiorespiratory effects of biphalin in anesthetized rats. Pharmacol Rep 63(5):1235–1242
Yamamoto T, Nair P, Davis P, Ma SW, Navratilova E, Moye S, Tumati S, Lai J, Vanderah TW, Yamamura HI, Porreca F, Hruby VJ (2007) Design, synthesis, and biological evaluation of novel bifunctional C-terminal-modified peptides for delta/mu opioid receptor agonists and neurokinin-1 receptor antagonists. J Med Chem 50(12):2779–2786
Yekkirala AS, Roberson DP, Bean BP, Woolf CJ (2017) Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16(8):545–564
Yezierski RP, Hansson P (2018) Inflammatory and neuropathic pain from bench to bedside: what went wrong? J Pain 19(6):571–588
Acknowledgments
We would like to thank Drs. Mark S. Kleven and Vincent Castagné for running the primary observation (Irwin) test.
Funding
This research was supported by a NIDA/SBIR R43 DA026653-01A1 to Biousian Biosystems, Inc. (R.P. and E.J.B.) and an NIAMS AR054975 to G.W.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Edward J. Bilsky is co-owner of BBI. Robin Polt is co-owner of BBI.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stevenson, G.W., Giuvelis, D., Cormier, J. et al. Behavioral pharmacology of the mixed-action delta-selective opioid receptor agonist BBI-11008: studies on acute, inflammatory and neuropathic pain, respiration, and drug self-administration. Psychopharmacology 237, 1195–1208 (2020). https://doi.org/10.1007/s00213-019-05449-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05449-z