Abstract
Rationale
Ketamine is used by preadolescent and adolescent humans for licit and illicit purposes.
Objective
The goal of the present study was to determine the effects of acute and repeated ketamine treatment on the unconditioned behaviors and conditioned locomotor activity of preadolescent and adolescent rats.
Methods
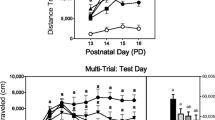
To assess unconditioned behaviors, female and male rats were injected with ketamine (5–40 mg/kg), and distance traveled was measured on postnatal day (PD) 21–25 or PD 41–45. To assess conditioned activity, male and female rats were injected with saline or ketamine in either a novel test chamber or the home cage on PD 21–24 or PD 41–44. One day later, rats were injected with saline and conditioned activity was assessed.
Results
Ketamine produced a dose-dependent increase in the locomotor activity of preadolescent and adolescent rats. Preadolescent rats did not exhibit sex differences, but ketamine-induced locomotor activity was substantially stronger in adolescent females than males. Repeated ketamine treatment neither caused a day-dependent increase in locomotor activity nor produced conditioned activity in preadolescent or adolescent rats.
Conclusions
The activity-enhancing effects of ketamine are consistent with the actions of an indirect dopamine agonist, while the inability of ketamine to induce conditioned activity is unlike what is observed after repeated cocaine or amphetamine treatment. This dichotomy could be due to ketamine’s ability to both enhance DA neurotransmission and antagonize N-methyl-D-aspartate (NMDA) receptors. Additional research will be necessary to parse out the relative contributions of DA and NMDA system functioning when assessing the behavioral effects of ketamine during early ontogeny.







Similar content being viewed by others
References
Ahmed SH, Cador M, Le Moal M, Stinus L (1995) Amphetamine-induced conditioned activity in rats: comparison with novelty-induced activity and role of the basolateral amygdala. Behav Neurosci 109:723–733
Ahmed SH, Oberling P, Di Scala G, Sandner G (1996) Amphetamine-induced conditioned activity does not result from a failure of rats to habituate to novelty. Psychopharmacology 123:325–332
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev 27:3–18
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79:565–575
Becker JB, Molenda H, Hummer DL (2001) Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci 937:172–187
Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G (2003) Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 27:687–700
Belujon P, Grace AA (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936
Bergman SA (1999) Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog 46:10–20
Botanas CJ, de la Peña JB, dela Peña IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH (2015) Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol Biochem Behav 133:31–36
Cameron DL, Crosbie J, Crocker AD (1988) A fixed interval momentary sampling method for assessing on-going behaviours induced by dopamine receptor agonists. Prog Neuro-Psychopharmacol Biol Psychi 12:595–606
Campbell BA, Lytle LD, Fibiger HC (1969) Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science 166:635–637
Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD (2016) Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther 359:159–170
Carey RJ, Damianopoulos EN, Shanahan AB (2008) Cocaine conditioned behavior: a cocaine memory trace or an anti-habituation effect. Pharmacol Biochem Behav 90:625–631
Caster JM, Walker QD, Kuhn CM (2007) A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology 193:247–260
Damianopoulos EN, Carey RJ (1992) Pavlovian conditioning of CNS drug effects: a critical review and new experimental design. Rev Neurosci 3:65–77
Damianopoulos EN, Carey RJ (1994) A new method to assess Pavlovian conditioning of psychostimulant drug effects. J Neurosci Methods 53:7–17
Dillon P, Copeland J, Jansen K (2003) Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend 69:23–28
Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA (1999) Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res 843:171–183
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 5:241–252
Franklin TR, Druhan JP (2000) Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology 23:633–644
Frantz KJ, O’Dell LE, Parsons LH (2006) Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 32:625–637
French ED, Ceci A (1990) Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett 119:159–162
Geisser S, Greenhouse SW (1958) An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Statist 29:885–891
Guo R, Tang Q, Ye Y, Lu X, Chen F, Dai X, Yan Y, Liao L (2016) Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regul Toxicol Pharmacol 77:263–274
Hancock PJ, Stamford JA (1999) Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth 82:603–608
Hodgson SR, Hofford RS, Buckman SAG, Wellman PJ, Eitan S (2010) Morphine-induced stereotyped thigmotaxis could appear as enhanced fear and anxiety in some behavioural tests. J Psychopharmacol 24:875–880
Irifune M, Shimizu T, Nomoto M (1991) Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 40:399–407
Jansen KLR (1993) Non-medical use of ketamine. Br Med J 306:601–602
Jansen KLR (2000) A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs 32:419–433
Johnson SA, Sediqzadah S, Erb S (2012) Expression and resilience of a cocaine-conditioned locomotor response after brief and extended drug-free periods. Behav Brain Res 230:69–77
Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE (2015) Monitoring the future national survey results on drug use: 1975–2014: overview, key findings on adolescent drug use. Ann Arbor, Institute for Social Research, The University of Michigan
Kalsi SS, Wood DM, Dargan PI (2011) The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4:7107
van der Kam EL, De Vry J, Tzschentke TM (2009) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. Eur J Pharmacol 606:94–101
Keita H, Lecharny JB, Henzel D, Desmonts JM, Mantz J (1996) Is inhibition of dopamine uptake relevant to the hypnotic action of i.v. anaesthetics? Br J Anaesth 77:254–256
Kohrs R, Durieux ME (1998) Ketamine: teaching an old drug new tricks. Anesth Analg 87:1186–1193
Lindefors N, Barati S, O’Connor WT (1997) Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res 759:205–212
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706
Mantz J, Varlet C, Lecharny JB, Henzel D, Lenot P, Desmonts JM (1994) Effects of volatile anesthetics, thiopental, and ketamine on spontaneous and depolarization-evoked dopamine release from striatal synaptosomes in the rat. Anesthesiology 80:352–363
McCormick CM, Mathews IZ (2007) HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav 86:220–233
McCormick CM, Green MR, Simone JJ (2017) Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol Stress 6:31–43
McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA (1999) Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharm 7:208–218
McDougall SA, Pipkin JA, Der-Ghazarian T, Cortez AM, Gutierrez A, Lee RJ, Carbajal S, Mohd-Yusof A (2014) Age-dependent differences in the strength and persistence of psychostimulant-induced conditioned activity in rats: effects of a single environment-cocaine pairing. Behav Pharmacol 25:695–704
McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA (2015) Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2High receptors. Psychopharmacology 232:2287–2301
Michel A, Tirelli E (2002) Post-sensitisation conditioned hyperlocomotion induced by cocaine is augmented as a function of dose in C57BL/6J mice. Behav Brain Res 132:179–186
Morgan CJ, Curran HV (2012) Ketamine use: a review. Addiction 107:27–38
Morikawa H, Paladini CA (2011) Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198:95–111
Nabeshima T, Yamaguchi K, Furukawa H, Kameyama T (1984a) Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats. Eur J Pharmacol 105:197–206
Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T (1984b) Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur J Pharmacol 97:217–227
National Research Council (2010) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington
Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M (1998) Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 88:768–774
Ojeda SR, Urbanski HF (1994) Puberty in the rat. In: Knobil R, Neill JD (eds) The physiology of reproduction, vol 2, 2nd edn. Raven Press, New York, pp 363–409
Parylak SL, Caster JM, Walker QD, Kuhn CM (2008) Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav 89:314–323
Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ (1999) Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res 101:15–20
Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. Behav Brain Res 140:1–47
Romeo RD, Patel R, Pham L, So VM (2016) Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev 70:206–216
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145
Sell SL, Scalzitti JM, Thomas ML, Cunningham KA (2000) Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther 293:879–886
Shalaby IA, Spear LP (1980) Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol 67:451–459
Shelnutt SR, Gunnell M, Owens SM (1999) Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J Pharmacol Exp Ther 290:1292–1298
Siegel S, Castellan NJ Jr (1988) Nonparametric statistics for the behavioral sciences, 2nd edn. McGraw-Hill, Boston
Simon P, Dupuis R, Costentin J (1994) Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 61:59–64
Smith RF (2003) Animal models of periadolescent substance abuse. Neurotoxicol Teratol 25:291–301
Smith DJ, Azzaro AJ, Zaldivar SB, Palmer S, Lee HS (1981) Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology 20:391–396
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Spear NE, McKenzie DL (1994) Intersensory integration in the infant rat. In: Lewkowicz DJ, Terrace H (eds) The development of intersensory perception: comparative perspectives. Erlbaum, Hillsdale, pp 133–161
Spear NE, Kraemer PJ, Molina JC, Smoller DE (1988) Developmental change in learning and memory: infantile disposition for unitization. In: Delacour J, Levy JCS (eds) Systems with learning and memory abilities. Elsevier, New York, pp 27–52
Tan S, Lam WP, Wai MS, Yu WH, Yew DT (2012) Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One 7:e43947
Treit D, Fundytus M (1989) Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav 31:959–962
Trujillo KA, Zamora JJ, Warmoth KP (2008) Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry 63:178–183
Tso MM, Blatchford KL, Callado LF, McLaughlin DP, Stamford JA (2004) Stereoselective effects of ketamine on dopamine, serotonin and noradrenaline release and uptake in rat brain slices. Neurochem Int 44:1–7
Turgeon SM, Anderson N, O’Loughlin K (2010) Phencyclidine (PCP) produces sexually dimorphic effects on voluntary sucrose consumption and elevated plus maze behavior. Pharmacol Biochem Behav 95:173–178
Uchihashi Y, Kuribara H, Morita T, Fujita T (1993) The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Japanese J Pharmacol 61:149–151
Usun Y, Eybrard S, Meyer F, Louilot A (2013) Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex. Behav Brain Res 256:229–237
Verma A, Moghaddam B (1996) NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16:373–379
Wessinger WD (1995) Sexual dimorphic effects of chronic phencyclidine in rats. Eur J Pharmacol 277:107–112
Wiley JL, Evans RL, Grainger DB, Nicholson KL (2011) Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacol Rep 63:1085–1092
Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, Sumner J (2005) Naloxone increases ketamine-induced hyperactivity in the open field in female rats. Pharmacol Biochem Behav 81:530–534
Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A (2007) Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav 91:202–207
Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82
Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP (1998) Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology 138:114–123
Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H (2016) Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci Lett 610:48–53
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Zavala AR, Nazarian A, Crawford CA, McDougall SA (2000) Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology 151:291–298
Zorrilla EP (1997) Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 30:141–150
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding sources
This research was supported by NIGMS training grant GM083883 (TJB and VR) and NIDA training grant DA033877 (AEM).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
McDougall, S.A., Moran, A.E., Baum, T.J. et al. Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacology 234, 2683–2696 (2017). https://doi.org/10.1007/s00213-017-4660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4660-3