Abstract
Rationale
Acute or chronic environmental enrichment (EE) reduces sucrose cue reactivity in rats. This effect may be mediated by dopamine receptors.
Objectives
We examined whether dopamine D1 or D2 receptor agonism could reverse the EE effect. We also examined whether any reversal effects would vary with the incubation of sucrose craving.
Methods
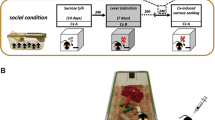
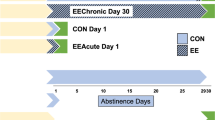
Following 10 days (2 h/day) of sucrose self-administration, rats experienced either 1 or 30 days of forced abstinence and either overnight (acute) or 29 day (chronic) EE. D1 (SKF 81297; 0, 0.3, or 1 mg/kg) or D2 (quinpirole; 0, 0.1, or 0.3 mg/kg) agonist was administered systemically immediately prior to a subsequent 2-h cue reactivity test the next day (n = 9–12 per group).
Results
Dose-dependent effects were limited to the day 1 test. High doses of the agonists increased day 1 acute EE cue reactivity to levels comparable to control animals. On the day 30 test, SKF 81297 increased cue reactivity in acute EE, chronic EE, and control rats. In contrast, quinpirole resulted in similar cue reactivity for control and enriched rats, more from a reduction in responding by controls vs. a recovery of responding by EE-experienced rats.
Conclusions
Both D1 and D2 receptors may be involved in the acute EE-mediated decrease in cue reactivity observed following 1 day of forced abstinence. In contrast, at 30 days of forced abstinence, D1 receptors may be critical in cue reactivity as SKF 81297 was effective at both restoring responding of enriched animals and potentiating responding of controls.




Similar content being viewed by others
References
Alexander BK (2010) The Globalization of Addiction. Oxford University Press.
Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB (1981) Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav 15:571–576
Alexander BK, Coambs RB, Hadaway PF (1978) The effect of housing and gender on morphine self-administration in rats. Psychopharmacology 58:175–179
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL (2002) Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 159:284–293
Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT (2012) Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol 23:650–657
Anghelescu I, Heuser I (2008) Psychostimulants. In: Offermanns S, Rosenthal W (eds) Encyclopedia of molecular pharmacology, 2nd edn. Springer, New York, pp. 1038–1044
Bowling SL, Rowlett JK, Bardo MT (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893
Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M (2009) Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34:2767–2778
Daniel JM, Roberts SL, Dohanich GP (1999) Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav 66:11–20
Darna M, Beckmann JS, Gipson CD, Bardo MT, Dwoskin LP (2015) Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res 1599:115–125
Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F (2007) Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm (Vienna) 114:43–48
du Hoffmann J, Nicola SM (2014) Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci 34:14349–14364
Ferland JM, Zeeb FD, Yu K, Kaur S, Taves MD, Winstanley CA (2014) Greater sensitivity to novelty in rats is associated with increased motor impulsivity following repeated exposure to a stimulating environment: implications for the etiology of impulse control deficits. Eur J Neurosci 40:3746–3756
Flaherty CF, Becker HC, Checke S, Rowan GA, Grigson PS (1992) Effect of chlorpromazine and haloperidol on negative contrast. Pharmacol Biochem Behav 42:111–117
Genn RF, Ahn S, Phillips AG (2004) Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci 118:869–873
Gerdjikov TV, Baker TW, Beninger RJ (2011) Amphetamine-induced enhancement of responding for conditioned reward in rats: interactions with repeated testing. Psychopharmacology 214:891–899
Gill KE, Beveridge TJ, Smith HR, Porrino LJ (2013) The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Res 1527:67–78
Grimm JW (2011) Animal models of craving. In: Olmstead C, Walz W (eds) Animal models of drug addiction. Humana Press, USA
Grimm JW (2012) Incubation of sucrose craving in animal models. In: Brownell K, Gold M (eds) Handbook of food and addiction. Oxford University Press, Oxford
Grimm JW, Barnes JL, Koerber J, Glueck E, Ginder D, Hyde J, Eaton L (2016) Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct Funct 221:2817–2830
Grimm JW, Buse C, Manaois M, Osincup D, Fyall A, Wells B (2006) Time-dependent dissociation of cocaine dose-response effects on sucrose craving and locomotion. Behav Pharmacol 17:143–149
Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S (2011) Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology 216:219–233
Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH (2008) Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol 19:777–785
Grimm JW, Weber R, Barnes J, Koerber J, Dorsey K, Glueck E (2013) Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration. PLoS One 8:e54164
Guy EG, Choi E, Pratt WE (2011) Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res 219:265–272
Heinla I, Leidmaa E, Visnapuu T, Philips MA, Vasar E (2014) Enrichment and individual housing reinforce the differences in aggressiveness and amphetamine response in 129S6/SvEv and C57BL/6 strains. Behav Brain Res 267:66–73
Hellemans KG, Benge LC, Olmstead MC (2004) Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res 150:103–115
Ikemoto S, Yang C, Tan A (2015) Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res 290:17–31
Kirkpatrick K, Marshall AT, Clarke J, Cain ME (2013) Environmental rearing effects on impulsivity and reward sensitivity. Behav Neurosci 127:712–724
Li X, Meng L, Huang K, Wang H, Li D (2015) Environmental enrichment blocks reinstatement of ethanol-induced conditioned place preference in mice. Neurosci Lett 599:92–96
Linthorst AC, De Lang H, De Jong W, Versteeg DH (1991) Effect of the dopamine D2 receptor agonist quinpirole on the in vivo release of dopamine in the caudate nucleus of hypertensive rats. Eur J Pharmacol 201:125–133
Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ (2014) Super-enrichment’ reveals dose-dependent therapeutic effects of environmental stimulation in a transgenic mouse model of Huntington’s disease. J Huntingtons Dis 3:299–309
Meyer AC, Bardo MT (2015) Amphetamine self-administration and dopamine function: assessment of gene x environment interactions in Lewis and Fischer 344 rats. Psychopharmacology 232:2275–2285
Mottola D, Kilts J, Lewis M, Connery H, Walker Q, Jones S, Booth R, Hyslop D, Piercey M, Wightman R, Lawler C (2002) Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther 301:1166–1178
Murtha S, Pappas BA, Raman S (1990) Neonatal and adult forebrain norepinephrine depletion and the behavioral and cortical thickening effects of enriched/impoverished environment. Behav Brain Res 39:249–261
Neumeyer J, Kula N, Bergman J, Baldessarini R (2003) Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol 474:137–140
Pham TM, Soderstrom S, Winblad B, Mohammed AH (1999) Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res 103:63–70
Phelps CE, Mitchell EN, Nutt DJ, Marston HM, Robinson ES (2015) Psychopharmacological characterisation of the successive negative contrast effect in rats. Psychopharmacology 232:2697–2709
Phillips AG, LePiane FG (1986) Effects of pimozide on positive and negative incentive contrast with rewarding brain stimulation. Pharmacol Biochem Behav 24:1577–1582
Salamone JD, Pardo M, Yohn S, López-Cruz L, SanMiguel N, Correa M (2016) Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci 27:231–257
Saunders BT, Yager LM, Robinson TE (2013) Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci 33:13989–14000
Self DW, Barnhart WJ, Lehman DA, Nestler EJ (1996) Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science 271:1586–1589
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Solinas M, Thiriet N, Chauvet C, Jaber M (2010) Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 92:572–592
Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL (2009) Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol 12:1151–1156
Torres C, Morales A, Candido A, Maldonado A (1996) Successive negative contrast in one-way avoidance: effect of thiopental sodium and chlorpromazine. Eur J Pharmacol 314:269–275
Uejima JL, Bossert JM, Poles GC, Lu L (2007) Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res 181:292–296
Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224:25–52
Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H (1977) Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psychol 91:33–50
Wise RA (1987) The role of reward pathways in the development of drug dependence. Pharmacol Ther 35:227–263
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Acknowledgements
The authors wish to thank Fiona Griffin, Neil Ingermann, Helena Reisterer, and Matthew Kroll for the help with the data collection. This study was supported by the National Institutes of Health grant DA016285-04 and Western Washington University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Glueck, E., Ginder, D., Hyde, J. et al. Effects of dopamine D1 and D2 receptor agonists on environmental enrichment attenuated sucrose cue reactivity in rats. Psychopharmacology 234, 815–825 (2017). https://doi.org/10.1007/s00213-016-4516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4516-2