Abstract
Rationale
Two biomarkers: concentration ratio of O-desmethylvenlafaxine/venlafaxine and concentration sum of venlafaxine + O-desmethylvenlafaxine were adopted to indicate venlafaxine responses, but neither is validated.
Objectives
To evaluate the ability of two biomarkers in reflecting venlafaxine pharmacokinetic variations, and to further examine their relationship with venlafaxine treatment outcomes.
Methods
Two well-defined influencing factors: CYP2D6 genotypes and drug interactions were enriched into a three-period crossover study to produce venlafaxine pharmacokinetic variations: In each period, healthy CYP2D6 extensive metabolizers (EM group; n = 12) and CYP2D6*10/*10 intermediate metabolizers (IM group; n = 12) were pretreated with clarithromycin (CYP3A4 inhibitor), or nothing (control), or clarithromycin + paroxetine (CYP3A4 + CYP2D6 inhibitors), before administration of a single-dose of 75 mg venlafaxine. Both biomarkers were evaluated (1) for their relationship with the influencing factors in healthy volunteers and (2) for their relationships with the venlafaxine responses/adverse events reported in two patient studies.
Results
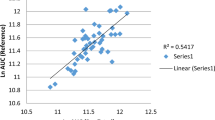
Significant venlafaxine pharmacokinetic variations were observed between the EM and IM groups (geometric mean ratio [95 % CI] of area under the curve, 3.0 [1.8–5.1] in the control period), and between the control and clarithromycin + paroxetine periods (4.1 [3.5–4.7] and 2.0 [1.7–2.4] in the EM and IM group, respectively). O-Desmethylvenlafaxine/venlafaxine was superior to venlafaxine + O-desmethylvenlafaxine to reflect the influencing factors. In the patient studies, O-desmethylvenlafaxine/venlafaxine > 4 showed high precision in predicting venlafaxine responders/partial-responders (92 %) and patients without venlafaxine-related adverse events (88 %); the O-desmethylvenlafaxine/venlafaxine < 4 and venlafaxine + O-desmethylvenlafaxine > 400 ng/ml combination showed higher precision (100 %) than O-desmethylvenlafaxine/venlafaxine < 4 alone (65 %) in predicting venlafaxine non-responders.
Conclusion
We propose using O-desmethylvenlafaxine/venlafaxine for CYP2D6 phenotyping, and O-desmethylvenlafaxine/venlafaxine with venlafaxine + O-desmethylvenlafaxine for predicting venlafaxine treatment outcomes in future prospective studies.






Similar content being viewed by others
References
Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kilic C, Offord D, Ustun TB, Wittchen HU (2003) The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) surveys. Int J Methods Psychiatr Res 12:3–21
Bachmeier CJ, Beaulieu-Abdelahad D, Ganey NJ, Mullan MJ, Levin GM (2011) Induction of drug efflux protein expression by venlafaxine but not desvenlafaxine. Biopharm Drug Dispos 32:233–244
Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitiello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, DeBar L, Keller M (2010) Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry 167:190–197
Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE (2012) Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther 92:467–475
de Silva VA, Hanwella R (2012) Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: a meta-analysis of published studies. Int Clin Psychopharmacol 27:8–16
Entsuah AR, Huang H, Thase ME (2001) Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry 62:869–877
Ereshefsky L, Dugan D (2000) Review of the pharmacokinetics, pharmacogenetics, and drug interaction potential of antidepressants: focus on venlafaxine. Depress Anxiety 12(Suppl 1):30–44
Flockhart D (2007) Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine
Fukuda T, Yamamoto I, Nishida Y, Zhou Q, Ohno M, Takada K, Azuma J (1999) Effect of the CYP2D6*10 genotype on venlafaxine pharmacokinetics in healthy adult volunteers. Br J Clin Pharmacol 47:450–453
Gex-Fabry M, Balant-Gorgia AE, Balant LP, Rudaz S, Veuthey JL, Bertschy G (2004) Time course of clinical response to venlafaxine: relevance of plasma level and chirality. Eur J Clin Pharmacol 59:883–891
Gu L, Xie J, Long J, Chen Q, Pan R, Yan Y, Wu G, Liang B, Tan J, Xie X, Wei B, Su L (2013) Epidemiology of major depressive disorder in mainland china: a systematic review. PLoS ONE 8:e65356
Hynninen VV, Olkkola KT, Bertilsson L, Kurkinen K, Neuvonen PJ, Laine K (2008) Effect of terbinafine and voriconazole on the pharmacokinetics of the antidepressant venlafaxine. Clin Pharmacol Ther 83:342–348
Karlsson L, Schmitt U, Josefsson M, Carlsson B, Ahlner J, Bengtsson F, Kugelberg FC, Hiemke C (2010) Blood–brain barrier penetration of the enantiomers of venlafaxine and its metabolites in mice lacking P-glycoprotein. Eur Neuropsychopharmacol 20:632–640
Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, Brockmoller J (2008) Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics 9:841–846
Lindh JD, Annas A, Meurling L, Dahl ML AAL-S (2003) Effect of ketoconazole on venlafaxine plasma concentrations in extensive and poor metabolisers of debrisoquine. Eur J Clin Pharmacol 59:401–406
Lobello KW, Preskorn SH, Guico-Pabia CJ, Jiang Q, Paul J, Nichols AI, Patroneva A, Ninan PT (2010) Cytochrome P450 2D6 phenotype predicts antidepressant efficacy of venlafaxine: a secondary analysis of 4 studies in major depressive disorder. J Clin Psychiatry 71:1482–1487
Lohoff FW, Narasimhan S, Rickels K (2013) Interaction between polymorphisms in serotonin transporter (SLC6A4) and serotonin receptor 2A (HTR2A) genes predict treatment response to venlafaxine XR in generalized anxiety disorder. Pharmacogenomics J 13:464–469
Macaluso M, Preskorn SH (2011) CYP 2D6 PM status and antidepressant response to nortriptyline and venlafaxine: is it more than just drug metabolism? J Clin Psychopharmacol 31:143–145
Mackinnon A (2000) A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput Biol Med 30:127–134
Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, Eddy SM, Dostalik J, Mount J, Azuma J, Fujio Y, Jang IJ, Shin SG, Bleavins MR, Williams JA, Paulauskis JD, Wilner KD (2008) Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 84:347–361
Ohayon MM, Hong SC (2006) Prevalence of major depressive disorder in the general population of South Korea. J Psychiatr Res 40:30–36
Patterson SD, Cohen N, Karnoub M, Truter SL, Emison E, Khambata-Ford S, Spear B, Ibia E, Sproule R, Barnes D, Bhathena A, Bristow MR, Russell C, Wang D, Warner A, Westelinck A, Brian W, Snapir A, Franc MA, Wong P, Shaw PM (2011) Prospective-retrospective biomarker analysis for regulatory consideration: white paper from the industry pharmacogenomics working group. Pharmacogenomics 12:939–951
PharmGKB (2011) Dutch Pharmacogenetics Working Group guideline for venlafaxine and CYP2D6. Pharmacogenomics Knowledge Base
Preskorn SH (2010) Understanding outliers on the usual dose–response curve: venlafaxine as a way to phenotype patients in terms of their CYP 2D6 status and why it matters. J Psychiatr Pract 16:46–49
Preskorn S, Patroneva A, Silman H, Jiang Q, Isler JA, Burczynski ME, Ahmed S, Paul J, Nichols AI (2009) Comparison of the pharmacokinetics of venlafaxine extended release and desvenlafaxine in extensive and poor cytochrome P450 2D6 metabolizers. J Clin Psychopharmacol 29:39–43
Preskorn SH, Kane CP, Lobello K, Nichols AI, Fayyad R, Buckley G, Focht K, Guico-Pabia CJ (2013) Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry 74:614–621
Sakolsky DJ, Perel JM, Emslie GJ, Clarke GN, Wagner KD, Vitiello B, Keller MB, Birmaher B, Asarnow JR, Ryan ND, McCracken JT, Strober MJ, Iyengar S, Porta G, Brent DA (2011) Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol 31:92–97
Shams ME, Arneth B, Hiemke C, Dragicevic A, Muller MJ, Kaiser R, Lackner K, Hartter S (2006) CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 31:493–502
Veefkind AH, Haffmans PM, Hoencamp E (2000) Venlafaxine serum levels and CYP2D6 genotype. Ther Drug Monit 22:202–208
Acknowledgments
This research was supported by a grant (11181MFDS655) from Ministry of Food and Drug Safety in 2011.
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hae-Deun Kim and Fen Jiang have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, F., Kim, HD., Na, HS. et al. The influences of CYP2D6 genotypes and drug interactions on the pharmacokinetics of venlafaxine: exploring predictive biomarkers for treatment outcomes. Psychopharmacology 232, 1899–1909 (2015). https://doi.org/10.1007/s00213-014-3825-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3825-6