Abstract
Atoguanil™ is a novel complex of atovaquone (ATV) and proguanil (PG) with enhanced ATV bioavailability compared to Malarone®. This pilot study assessed whether the relative bioavailability (Frel) of ATV, PG, and the primary PG metabolite cycloguanil (CG) following a single oral dose in the fed state of Atoguanil was similar to Malarone despite a 50% lower ATV dose. This open-label, single-dose, randomized 2-period, 2-treatment, balanced crossover study was conducted between 17th November 2021 and 18th March 2022. Eligible participants (aged 18–55 years) were randomized (1:1) in period 1 to Atoguanil (ATV/PG 500/348 mg) or Malarone (ATV/PG hydrochloride 1000/400 mg) administered following a high-fat, high caloric meal. After a 24-day washout period, participants crossed treatment arms. For the doses tested, Frel was assumed similar if 90%CIs were between 80 and 125% for the geometric mean ratio of the least square mean differences for each exposure parameter. In 15 evaluable participants, Frel was similar for ATV Cmax (93.6% [90%CI 83.6, 104.9]) but not AUC0-inf (77.8% [67.4, 89.8]), for PG AUC0-inf (95.6% [92.1, 99.2]) but not Cmax (82.4% [75.8, 89.5]), and for both CG Cmax (100.8% [95.0, 107.0]) and AUC0-inf (102.9% [98.4, 107.7]). Nine adverse events occurred; all were of mild severity and not considered treatment related. At the doses tested, ATV Frel was lower following Atoguanil versus Malarone based on AUC0-inf, though when adjusted for dose Frel increased by 156%. Both drugs were well tolerated with no safety concerns. ClinicalTrials.gov: NCT04866602 (April 26th, 2021)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most deaths from malaria occur in African children under 5 years of age with Plasmodium falciparum infection (World Health Organization 2023). Thus, preventing P. falciparum malaria in children is a key intervention in reducing the risk of mortality and morbidity associated with severe malaria.
The World Health Organization recommends seasonal malaria chemoprevention (SMC) for children aged 3 months to 5 years in areas with highly seasonal malaria transmission (World Health Organization 2022). A monthly dose of sulfadoxine-pyrimethamine + amodiaquine (SPAQ) is administered for 3 to 4 consecutive months to reduce malaria cases, hospitalizations, and deaths (Adjei et al. 2022). SMC deployment is currently limited to the sub-Sahel region due to a high prevalence of SPAQ-resistant P. falciparum in southern, central, and eastern Africa (Amimo et al. 2020). Moreover, the increased prevalence of molecular markers of drug resistance in areas where SPAQ is currently deployed for SMC is concerning (Ndiaye et al. 2023).
The antimalarial drug Malarone® is a fixed-dose combination of atovaquone (ATV) and proguanil (PG), formulated as the hydrochloride salt (PGHCl). The combination is active against P. falciparum liver and blood stages, being registered for P. falciparum malaria prophylaxis and the treatment of uncomplicated malaria or uncomplicated P. falciparum malaria (Croft 2010; Nakato et al. 2007; Blanshard and Hine 2021). Malarone has a favorable safety profile and good tolerability (Nakato et al. 2007; Blanshard and Hine 2021; Bustos et al. 1999; Overbosch 2003). Despite the patent for Malarone expiring in 2013, generic ATV-PGHCl has not been considered for use in chemoprevention in malaria endemic regions because of its high cost.
The hydroxynaphthoquinone ATV is a competitive inhibitor of ubiquinol, disrupting the parasite mitochondrial electron transport chain at the bc1 complex. Elimination is mainly via the liver through bile clearance (Zsila and Fitos 2010), with no evidence of metabolization and an elimination half-life of ~50–84 h (Rolan et al. 1997; Nixon et al. 2013). ATV is highly protein bound (> 99.5%) and lipophilic (log P 5.1) with poor oral bioavailability (Nixon et al. 2013). Administration with a high-fat meal increases the area under the concentration–time curve (AUC) by 3.3-fold and maximum concentration (Cmax) by 5.3-fold (Rolan et al. 1994). However, even when taken with food, as per the Malarone label recommendation, ATV maximum bioavailability was 23% (Hussein et al. 1996). Because of the low exposures achieved, ATV monotherapy has limited efficacy and parasite resistance readily emerges (Looareesuwan et al. 1996). However, antimalarial efficacy is enhanced synergistically when ATV is combined with PG, reducing the risk of recrudescence and ATV resistance emergence (Looareesuwan et al. 1996; Lin et al. 2021). Nevertheless, the high ATV dose and a relatively expensive manufacturing process results in a high cost-of-goods for Malarone, limiting its use for malaria prevention in endemic countries.
Proguanil is a biguanide derivative that is converted to 4-chlorophenyl biguanide and the active metabolite cycloguanil (CG), a parasite dihydrofolate reductase (DHFR) inhibitor. Metabolism is via cytochrome P450 (CYP) 2C19 and CYP3A4 (Pudney et al. 1999). Genetic polymorphism in CYP2C19 results in higher PG and lower CG plasma concentrations in poor metabolizers (Beerahee 1999). The synergy between ATV and PG does not appear to result from DHFR inhibition. Rather, PG enhances the ATV provoked collapse in mitochondrial membrane potential without affecting electron transport inhibition (Srivastava and Vaidya 1999). This explains why ATV-PGHCl does not require dose adjustment in populations with a high prevalence of poor PG metabolizers and retains efficacy against PG-resistant parasites (Hussein et al. 1996; Srivastava and Vaidya 1999).
To enhance ATV bioavailability and potentially reduce the required dose, a novel complex of ATV with PG free base has been developed (Atoguanil™). Atoguanil dissociates into ATV and PG in vivo when it comes into contact with gastro-intestinal fluids. The resulting ATV is found to be more polar and more soluble in aqueous media compared to free ATV from Malarone, resulting in better absorption and bioavailability in vivo. ATV from Atoguanil showed a more than three-fold higher dissolution (over 90%) compared to ATV from Malarone. In a preclinical pharmacokinetic study in the rabbit, this difference in dissolution translated into an approximately twofold increase in ATV oral bioavailability with Atoguanil compared to Malarone, and similar findings were reported in rats (Medicines for Malaria Venture, data on file).
This pilot study in healthy participants compared the pharmacokinetic (PK) profiles and drug exposures for ATV, PG, and CG obtained after Atoguanil or Malarone administration, containing an ATV dose of 500 mg or 1000 mg, respectively. The aim was to determine whether a twofold reduction in the ATV dose with Atoguanil was feasible while maintaining appropriate therapeutic exposure. Such an improved bioavailability would potentially lead to a significant cost reduction with Atoguanil versus Malarone, increasing affordability for use in malaria chemoprevention, including SMC, in endemic regions.
Methods
Study design
This open-label, single-dose, randomized 2-period, 2-treatment balanced crossover pilot study evaluated the PK profile and relative bioavailability (Frel) of single-dose Atoguanil tablets in healthy adults relative to Malarone in the fed state.
Study drugs were Atoguanil (Ipca Laboratories Ltd, Mumbai, India), comprising ATV 500 mg plus PG free base 348 mg administered as four tablets of 125:87 mg; and Malarone (GSK, Ware, UK) comprising ATV 1000 mg plus PGHCl 400 mg (equivalent to PG 348 mg free base) administered as four tablets of 250:100 mg. The Malarone dose administered was the approved dose for treating acute uncomplicated malaria as chemoprevention currently requires a full course of antimalarial treatment (World Health Organization 2022). The Atoguanil ATV dose was reduced from 1000 to 500 mg, anticipating similar ATV exposures after oral administration of Atoguanil and Malarone. Exposure to PG from Atoguanil was predicted to be similar to that from Malarone. Thus, PG dose (free base) in Atoguanil matched the PG dose (hydrochloride) in Malarone.
As Malarone is administered with food or a milky drink, the fed condition was selected to assess bioavailability for both formulations. The study was conducted at Richmond Pharmacology Ltd (London, UK) between 17th November 2021 and 18th March 2022 (ClinicalTrials.gov: NCT04866602 registered 26th April, 2021; EudraCT number 2021-003422-69).
The primary objective was to determine the Frel of ATV, PG, and CG derived from a single oral dose of Atoguanil compared to a single oral dose of Malarone in the fed state at the doses administered. Secondary objectives were to further describe the single-dose PK properties of ATV, PG, and CG in healthy participants following a single dose of Atoguanil or Malarone in the fed state, to assess the safety and tolerability of Atoguanil, and further document the safety/tolerability of Malarone.
The study conformed to Good Clinical Practice as per the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines, the Declaration of Helsinki, and all applicable local laws and guidance. All participants provided informed signed consent before undertaking any study procedure. The study protocol including one amendment were reviewed and approved by the London Bridge Research Ethics Committee.
Participants
Eligible participants were male and female, including those of child-bearing potential, aged between 18 and 55 years, with bodyweight 50 to 80 kg and a body mass index 18 to 25 kg/m2. Participants were in good health, non-smokers, with no significant medical history, or clinically relevant abnormalities at screening based on physical examination, vital signs, clinical laboratory evaluations, or electrocardiogram (ECG). All participants had to use highly effective contraception, unless post-menopausal or sterilized. Pregnant and lactating women or partners of pregnant or lactating women were excluded. Key exclusion criteria were known hypersensitivity to ATV or PG, any significant underlying disease, condition or infection, including HIV, hepatitis B and drug and food allergies, current or history of psychiatric illness, any condition affecting drug absorption, a history of photosensitivity, a history or clinical evidence of substance or alcohol abuse, receipt of an investigational drug within 90 days or 5 half-lives, any moderate/strong inhibitor/inducer of CYP450 within 30 days or 5 half-lives, or any other drug in the previous 7 days or 10 half-lives or herbal supplement within 30 days, or any other significant disease or disorder considered to put the participant at risk.
Procedures
Participants were screened within 30 days of trial start (Day −1). In period 1, participants were randomized (1:1) to Atoguanil or Malarone according to a computer-generated randomization schedule with cross-over to the other treatment in period 2. For each study period, study medication was administered on Day 1, participants discharged on Day 4, and outpatient follow-up visits conducted on Days 8, 15, and 22. There was a minimum 24-day washout period between each study period. Post-screening procedures are shown in Table 1.
Before treatment administration, participants fasted overnight for at least 10 h, then consumed an FDA-type high-fat/calorie breakfast (796 kcal, 45.2 g/406.8 kcal of fat). Study drug was administered at completion of the breakfast being consumed entirely within 30 min of serving and with 240 mL of water. No additional food was allowed for 4 h post-dose, and additional water intake was not allowed 1 h before and after dosing. Participants were asked to not lie fully recumbent for 2 h post-dosing. Blood samples were collected for determination of ATV, PG, and CG plasma concentrations using a validated method. The lower limit of quantification (LLOQ) was 100 ng/mL for ATV, 5.00 ng/mL for PG and 1.50 ng/mL for CG.
Endpoints
The primary endpoints were Frel for ATV at the doses tested for Cmax, the AUC from time zero to last detectable plasma concentration (AUC0-t), AUC from time zero to 72 h (AUC0-72h), AUC from time zero to 168 h (AUC0-168h), and AUC from time zero to infinity (AUC0-inf). Also, the Frel for PG and CG for Cmax and AUC0-inf at the doses tested were primary endpoints. Although AUC0-inf is recommended as the most relevant measure for assessing Frel (United States Food and Drug Administration 2022), as ATV has a long terminal half-life (t1/2), additional AUC endpoints were considered in case AUC0-inf could not be calculated.
Secondary PK endpoints were time to maximum plasma concentration (Tmax), terminal rate constant (λz), terminal half-life (t1/2), apparent volume of distribution during the terminal phase (Vz/F), apparent total plasma clearance (Cl/F), and percentage of AUC due to extrapolation from the last measured value to infinity (%AUCextrap).
Safety endpoints were the frequency of treatment-emergent adverse events, serious adverse events and adverse events of special interest, and the proportion of participants with clinically relevant changes in laboratory safety tests, vital signs (supine), or ECG (as triplicate) parameters. Adverse events of special interest were alanine transaminase (ALT) or aspartate transaminase (AST) > 3× the upper limit of normal (ULN) plus total bilirubin > 1.5×ULN, ALT or AST >8×ULN, or >3×ULN and symptomatic, uncorrected QT interval prolongation >500 msec, decline in hemoglobin ≥ 25% from baseline or an absolute value < 10 g/dL, clinically relevant decrease in neutrophil count, and platelet count ≥ 25% from baseline or an absolute value < 80 × 109/L.
Statistical analysis
For this pilot study with first administration of Atoguanil in humans, no formal statistical power calculation was done, and 16 participants were enrolled to have at least 14 participants complete. A previous assessment of Malarone PK in healthy adult participants indicated intersubject coefficient of variation (CV%) values of 20–23% for ATV and PG Cmax and PG AUC0-inf, but > 30% for ATV AUC0-inf, suggesting that the proposed sample size would not allow for a formal bioequivalence assessment (Beerahee 1999). Thus, this study was conducted as an exploratory investigation to inform the design of a subsequent formal bioequivalence study.
The safety population included all randomized participants who received at least one dose of study treatment and was used for the safety analyses. The PK population was used for the PK analyses and included participants in the safety population with sufficient blood samples for calculation of at least one PK parameter.
PK parameters were estimated using non-compartmental analysis based on individual plasma concentration data, and the actual time of drug administration and blood sampling using Phoenix WinNonlin Version 8.3 (Certara, St Louis, USA). Samples below the LLOQ prior to the first quantifiable concentration were set to zero and those after the first quantifiable concentration were set to missing and omitted from the analysis. AUC was calculated using the linear/log trapezoidal method, applying the linear trapezoidal rule up to Cmax and the log trapezoidal rule for the remainder of the curve. Other PK parameters were calculated according to standard equations and summarized using descriptive statistics.
Frel at the doses tested was assessed as the ratio of the geometric means for exposure parameters expressed as a percentage, i.e., (Atoguanil/Malarone) × 100%. Frel was calculated for ATV using for Cmax, AUC0–t, AUC0–72h, AUC0–168h, and AUC0–inf and for PG and CG using Cmax and AUC0-inf. Using the bioequivalence module within Phonenix WinNonlin, a linear mixed effect model was used to obtain the geometric means ratios, with the logarithm of the PK parameter as the response variable, the sequence, treatment, and period as fixed effects, and the subject within sequence as the random effect. Least square mean differences (Atoguanil − Malarone) were extracted from the model with 90% confidence intervals (CI). Mean ratios were reported with two-sided 90%CI after back transformation from the log-scale. For this pilot study, bioavailability was assumed to be similar if the 90%CI limits for the Frel for each exposure parameter did not exceed 80% to 125%. A post hoc analysis was performed to determine dose-adjusted Frel as the ratio of the geometric means for exposure parameters expressed as a percentage following formula: ((Atoguanil×1000)/(Malarone×500)) ×100%.
Adverse events were classified using the Medical Dictionary for Regulatory Activities (MedDRA, version 24.1). Adverse event severity was classified using a categorical grading system (mild/moderate/severe) based on a global clinical assessment by a trained research physician. All safety endpoints were analyzed using descriptive statistics using SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Results
Participants
Of 38 individuals screened, 22 were excluded (5 reserve participants, 7 laboratory findings, 7 withdrew consent, 4 BMI/weight, 1 ECG, 1 medical history; including 3 re-screenings). All 16 enrolled participants completed the study and were included in the safety and PK populations. Mean (SD) age was 27.8 (4.9) years and 50% (8/16) were female; all females were of child-bearing potential (Table 2). The pre-specified threshold for a period 2 pre-dose ATV concentration > 5% of Cmax was observed for one participant, who was excluded from the analysis of Frel for both periods (modified PK population of 15 subjects). There were no other exclusions.
Pharmacokinetics
PK parameters
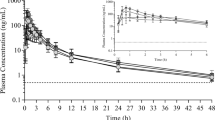
Following peak ATV plasma concentrations, plasma levels declined in a multiphasic manner and remained quantifiable up to 504 h post-dose in both treatment groups (Fig. 1). Measurable concentrations of PG and CG were present until at least 72 h post-dose with a Tlag of between 0.13 and 0.25 h with PG and 1.00 and 0.72 h for CG, reflecting the rapid metabolic conversion (Fig. 1). Median Tmax of ATV after administration of Atoguanil and Malarone was 4.5 h for both products. PK parameters for ATV, PG, and CG following Atoguanil or Malarone are summarized in Table 3.
Mean (SD) plasma concentrations for ATV, PG, and CG over time following oral administration of Atoguanil (ATV 500 mg PG 348 mg) or Malarone (ATV 1000 mg plus PG hydrochloride 400 mg) in the presence of food (PK population, n = 16). ATV, atovaquone; PG, proguanil; CG, cycloguanil; SD, standard deviation
Relative bioavailability
Geometric means and intersubject variability (CV%) and the analysis of Frel for PK exposure measures are shown in Table 4. At the doses tested, Malarone treatment gave rise to higher ATV exposure values when compared to Atoguanil with moderate CV%. ATV Frel following Atoguanil was 93.6% (90%CI 83.6, 104.9) for Cmax and 77.8% (90%CI 67.4, 89.8) for AUC0-inf. Thus, in this pilot study, at the doses tested Frel between Atoguanil 500 mg and Malarone 1000 mg was similar for ATV Cmax, but not for AUC0-inf. When correcting for the ATV dose, based on AUC0-inf the Frel of Atoguanil was 156% compared to Malarone.
At the doses tested, PG Cmax was lower following Atoguanil versus Malarone (82.4% 90%CI [75.8, 89.5]), though AUC0-inf values were similar for the two formulations (Table 4). PK exposure parameters for CG were similar for Atoguanil and Malarone (Table 4).
Safety
Following Atoguanil, four adverse events (3 COVID-19 infection, 1 soft tissue infection) occurred in 25.0% (4/16) of participants. Following Malarone, five adverse events (1 diarrhea, 3 headache, 1 contact dermatitis) occurred in 25.0% (4/16) of participants. All adverse events were of mild severity, two cases of headache were treated symptomatically with fluid/analgesics, and all resolved by period end. None of the adverse events was considered related to study medication. There were no serious adverse events or deaths. There were no adverse events of special interest reported.
There were no clinically significant changes in biochemistry, hematology, or coagulation parameters. There were no clinically relevant changes in ECG parameters. One participant receiving Malarone had a single reading of a transient prolongation of Fridericia-corrected QT interval of 454 msec (53 msec increase from baseline) at 6 h post-dose on Day 1, which was not observed in nine repeated readings and not considered clinically relevant. No clinically relevant observations were made for physical examination or vital signs.
Discussion
This open-label, single-dose, standard non-replicated randomized 2-period, 2-treatment balanced crossover pilot study evaluated the oral PK profile and Frel of a novel formulation of ATV-PG (Atoguanil) relative to ATV-PGHCl (Malarone) in healthy adults in the fed state. In vitro dissolution data of Malarone vs Atoguanil tablets in two discriminating dissolution media showed dissolution rates for Atoguanil were about 2 to 2.5 times that of Malarone (Medicines for Malaria Venture, data on file). Also, a study in rabbits showed similar bioavailability of 50 mg/kg Atoguanil to 100 mg/kg Malarone with the ratio for ATV AUC0-inf being 99.6% (95%CI 96.4, 103.0) (personal communication, Byju Thankachen). Thus, the maximum theoretical systemic exposure to ATV from Atoguanil was expected to be approximately twofold higher than for the Malarone. In this study, the proposed Atoguanil ATV dose was 500 mg, anticipating similar ATV exposures after oral administration of the Malarone ATV dose of 1000 mg.
PK parameters for ATV, PG, and CG following Malarone were consistent with previous reports in healthy participants in the fed state (Gillotin et al. 1999). At the doses tested, Frel was similar between Atoguanil and Malarone for Cmax (93.6% [90%CI 83.6, 104.9]), but not for AUC0-inf (77.8% [90%CI 67.4, 89.8]). The PG dose in Atoguanil was similar to that in Malarone, and PG and CG exposures were expected to be similar. This was the case except for the PG Cmax, with an Frel of 82.4% (95%CI 75.8, 89.5).
The preferred study design for evaluating the Frel is the cross-over study design. As ATV has a half-life of up to 116 h, a minimum wash-out interval of 24 days between the administration of two single doses was used. However, one participant had an ATV concentration > 5% of Cmax in the second period and data from both periods were excluded from the Frel analysis (modified PK data set).
The individual ATV concentration–time profiles following Malarone showed some transient plateau and secondary peaks after 24 h, observed nominally around the time the participants would be administered food. This observation may account for the higher ATV AUC following administration of Malarone versus Atoguanil. However, as the ATV dose in Atoguanil was 500 mg versus 1000 mg in Malarone, the dose-adjusted Frel for ATV was improved by 156% with Atoguanil based on AUC0-inf. The ATV AUC0-inf following administration of Atoguanil also showed a reduced variability compared to Malarone, with CV% values of 26.8% and 48.7%, respectively.
Only nine treatment-emergent adverse events were reported, with no apparent differences observed between the treatment groups. All adverse events were mild and not considered treatment related. No clinically relevant abnormalities were observed for clinical laboratory tests, hematology, vital signs, or ECG. Overall, the Atoguanil safety and tolerability profile was acceptable and comparable to that of Malarone.
Key limitations were that this is a pilot study with moderate intersubject variability observed for exposure parameters. The ATV AUC0-inf CV% observed in our study was largely above 30%, confirming previous data in healthy participants (Beerahee 1999). This suggests that a replicate design in which the reference product is given more than once, or even a two-stage design, would probably be required for a formal bioequivalence study (United States Food and Drug Administration 2022). According to the European Medicines Agency guidelines, widening the conventional 20% acceptance range for bioequivalence based on high variability is possible for Cmax but not AUC, and only if a replicate design is conducted (Committee for Medicinal Products for Human Use 2010). In contrast, the US Food and Drug Administration allows widening of the bioequivalence acceptance criteria for both Cmax and AUC, as well as applying slightly different approaches to estimate within-subject variation for the reference product above 25% (Endrenyi and Tothfalusi 2019). Importantly, this study used a high-fat, high calorific meal. As there was moderate intersubject variability in ATV exposures following Malarone and transitory secondary peaks in ATV concentrations around the time the participants were administered food, the possibility that there would be similar Frel between Atoguanil and Malarone following a low-fat meal or a milky drink as per normal Malarone dosing recommendations cannot be discounted, but this was not evaluated in this study. In terms of relevance to the target population, perennial malaria chemoprevention is recommended for children up to 24 months of age, and SMC is recommended for children under 5 years old. However, the safety and effectiveness of Malarone has not been confirmed in children weighing less than 11 kg. This study was necessarily conducted in healthy adults for ethical reasons.
Conclusions
In this pilot study, the ATV Frel following Atoguanil (ATV 500 mg PG 348 mg) or Malarone (ATV 1000 mg plus PG hydrochloride 400 mg) at the doses tested was not similar when both treatments were administered with high-fat/high caloric meal, based on AUC0-inf. However, given that Malarone contains twice the ATV dose of Atoguanil, the dose-adjusted Frel of Atoguanil was 156% compared to Malarone. Both drugs were well tolerated with no safety concerns. However, the feasibility of halving the ATV dose required with the Atoguanil formulation compared with Malarone was not established.
Data availability
De-identified participant data are available on reasonable request and with completion of a signed data access agreement from (https://www.mmv.org/about-us/contact-us) referencing this publication. Data will be available for at least five years from publication of this study.
References
Adjei MR, Kubio C, Buamah M, Sarfo A, Suuri T, Ibrahim S, Sadiq A, Abubakari II, Baafi JV (2022) Effectiveness of seasonal malaria chemoprevention in reducing under-five malaria morbidity and mortality in the Savannah Region. Ghana. Ghana Med J 56(2):64–70. https://doi.org/10.4314/gmj.v56i2.2
Amimo F, Lambert B, Magit A, Sacarlal J, Hashizume M, Shibuya K (2020) Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: a systematic analysis of national trends. BMJ Glob Health 5(11):e003217. https://doi.org/10.1136/bmjgh-2020-003217
Beerahee M (1999) Clinical pharmacology of atovaquone and proguanil hydrochloride. J Travel Med 6(Suppl 1):S13-17
Blanshard A, Hine P (2021) Atovaquone-proguanil for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev 1(1):CDOO4529. https://doi.org/10.1002/14651858.CD004529.pub3
Bustos DG, Canfield CJ, Canete-Miguel E, Hutchinson DB (1999) Atovaquone-proguanil compared with chloroquine and chloroquine-sulfadoxine-pyrimethamine for treatment of acute Plasmodium falciparum malaria in the Philippines. J Infect Dis 179(6):1587–1590. https://doi.org/10.1086/314770
Committee for Medicinal Products for Human Use (2010) Guideline on the investigation of bioequivalence. European Medicines Agency, London. Accessed: 2023 (29 November). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf
Croft AM (2010) Malaria: prevention in travellers. BMJ Clin Evid 2010
Endrenyi L, Tothfalusi L (2019) Bioequivalence for highly variable drugs: regulatory agreements, disagreements, and harmonization. J Pharmacokinet Pharmacodyn 46(2):117–126. https://doi.org/10.1007/s10928-019-09623-w
Gillotin C, Mamet JP, Veronese L (1999) Lack of a pharmacokinetic interaction between atovaquone and proguanil. Eur J Clin Pharmacol 55(4):311–315. https://doi.org/10.1007/s002280050634
Hussein Z, Eaves CJ, Hutchinson DB, Canfield CJ (1996) Population pharmacokinetics of proguanil in patients with acute P. falciparum malaria after combined therapy with atovaquone. Br J Clin Pharmacol 42(5):589–597. https://doi.org/10.1111/j.1365-2125.1996.tb00114.x
Lin JT, Waltmann A, Moser KA, Park Z, Na YB, Aydemir O, Brazeau NF, Gosi P, Marsh PW, Muller MS, Spring M, Sok S, Bailey JA, Saunders DL, Lon C, Wojnarski M (2021) Selection of cytochrome b mutants is rare among Plasmodium falciparum patients failing treatment with atovaquone-proguanil in Cambodia. Antimicrob Agents Chemother 65(3). https://doi.org/10.1128/AAC.01249-20
Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ (1996) Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg 54(1):62–66. https://doi.org/10.4269/ajtmh.1996.54.62
Nakato H, Vivancos R, Hunter PR (2007) A systematic review and meta-analysis of the effectiveness and safety of atovaquone proguanil (Malarone) for chemoprophylaxis against malaria. J Antimicrob Chemother 60(5):929–936. https://doi.org/10.1093/jac/dkm337
Ndiaye YD, Wong W, Thwing J, Schaffner SS, Tine A, Diallo MA, Deme A, Sy M, Bei AK, Thiaw AB, Daniels R, Ndiaye T, Gaye A, Ndiaye IM, Toure M, Gadiaga N, Sene A, Sow D, Garba MN, Yade MS, Dieye B, Diongue K, Zoumarou D, Ndiaye A, Gomis J, Fall FB, Ndiop M, Diallo I, Sene D, Macinnis B, Seck MC, Ndiaye M, Badiane AS, Hartl DL, Volkman SK, Wirth DF, Ndiaye D (2023) Two decades of molecular surveillance in Senegal reveal changes in known drug resistance mutations associated with historical drug use and seasonal malaria chemoprevention. medRxiv. https://doi.org/10.1101/2023.04.24.23288820
Nixon GL, Moss DM, Shone AE, Lalloo DG, Fisher N, O’Neill PM, Ward SA, Biagini GA (2013) Antimalarial pharmacology and therapeutics of atovaquone. J Antimicrob Chemother 68(5):977–985. https://doi.org/10.1093/jac/dks504
Overbosch D (2003) Post-marketing surveillance: adverse events during long-term use of atovaquone/proguanil for travelers to malaria-endemic countries. J Travel Med 10 Suppl 1: S16-20; discussion S21-13 https://doi.org/10.2310/7060.2003.35079
Pudney M, Gutteridge W, Zeman A, Dickins M, Woolley JL (1999) Atovaquone and proguanil hydrochloride: a review of nonclinical studies. J Travel Med 6(Suppl 1):S8-12
Rolan PE, Mercer AJ, Weatherley BC, Holdich T, Meire H, Peck RW, Ridout G, Posner J (1994) Examination of some factors responsible for a food-induced increase in absorption of atovaquone. Br J Clin Pharmacol 37(1):13–20. https://doi.org/10.1111/j.1365-2125.1994.tb04232.x
Rolan PE, Mercer AJ, Tate E, Benjamin I, Posner J (1997) Disposition of atovaquone in humans. Antimicrob Agents Chemother 41(6):1319–1321. https://doi.org/10.1128/AAC.41.6.1319
Srivastava IK, Vaidya AB (1999) A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother 43(6):1334–1339. https://doi.org/10.1128/AAC.43.6.1334
United States Food and Drug Administration (2022) Bioavailability studies submitted in NDAs or INDs – general considerations. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring. Accessed: 2023 (29 November). Available at: https://www.fda.gov/media/121311/download
World Health Organization (2022) WHO guidelines for malaria. WHO, Geneva. Accessed: 2023 (28 February). Available at: https://www.who.int/publications/i/item/guidelines-for-malaria
World Health Organization (2023) World Malaria Report 2023. WHO, Geneva. Accessed: 2024 (6 February). Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023
Zsila F, Fitos I (2010) Combination of chiroptical, absorption and fluorescence spectroscopic methods reveals multiple, hydrophobicity-driven human serum albumin binding of the antimalarial atovaquone and related hydroxynaphthoquinone compounds. Org Biomol Chem 8(21):4905–4914. https://doi.org/10.1039/c0ob00124d
Acknowledgements
The authors acknowledge the support of André-Marie Tchouatieu, Helen Demarest, from Medicines for Malaria Venture, Fraser Peck and Lucy Fulford Smith from Richmond Pharmacology Ltd, and Susan Podmore, independent consultant. Naomi Richardson (Magenta Communications Ltd) wrote the first draft of this article and provided editorial and graphic design services and was funded by Medicines for Malaria Venture.
Funding
Open access funding provided by Medicines for Malaria Venture MMV Funding for the conduct of this study and support for preparation of this manuscript was provided by Medicines for Malaria Venture. This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation (grant number INV-007155). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Author information
Authors and Affiliations
Contributions
Conceptualization: Andrea Kuemmerle, Myriam El Gaaloul, Ulrike Lorch, Ashok Kumar, Dharmendra Singh, Satinder Singh, Hanu Ramachandruni, Byju Thankachen, Swapnil Kore, Isabelle Borghini-Fuhrer, Stephan Chalon; methodology: Andrea Kuemmerle, Ulrike Lorch, Hanu Ramachandruni, Isabelle Borghini-Fuhrer, Stephan Chalon; formal analysis and investigation: Andrea Kuemmerle, Denis Gossen, Michael Marx, Ulrike Lorch, Maja Szramowska, Isabelle Borghini-Fuhrer, Stephan Chalon; writing—review and editing: Andrea Kuemmerle, Denis Gossen, Michael Marx, Ulrike Lorch, Maja Szramowska, Ashok Kumar, Dharmendra Singh, Satinder Singh, Hanu Ramachandruni, Byju Thankachen, Swapnil Kore, Myriam El Gaaloul, Isabelle Borghini-Fuhrer, Stephan Chalon; resources: Ulrike Lorch, Maja Szramowska, Byju Thankachen, Dharmendra Singh, Ashok Kumar, Swapnil Kore; supervision: Andrea Kuemmerle, Myriam El Gaaloul, Ulrike Lorch, Isabelle Borghini-Fuhrer, Stephan Chalon. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
All participants provided informed signed consent before undertaking any study procedure. The study protocol including one amendment were reviewed and approved by the London Bridge Research Ethics Committee.
Competing interests
HR, MEG, IB-F, and SC are employees of MMV. AK was employed at Medicines for Malaria Venture (MMV) when the study was designed and conducted and is a current employee of Novartis, Biomedical Research, Basel, Switzerland; the content of this paper is the responsibility of the individual authors and neither the study nor this publication is associated with Novartis, Biomedical Research. DG is the owner and director of Mangareva SRL, which received financial support from MMV to review and interpret the study results. MM is an employee of ICON Clinical Research, Langen, Germany, which received financial support from MMV to monitor the study. UL is an employee of Richmond Pharmacology Ltd, which received financial support from MMV to conduct the study. MS is an employee of PharmaKinetic Ltd which received financial support from MMV to perform the pharmacokinetic analysis. AK, DS, BT, and SK are employees of IPCA Laboratories Limited, Mumbai, India. SS was employed at IPCA Laboratories Limited when the study was designed and conducted and is a current employee of Aragen Life Sciences, Hyderabad, India; the content of this paper is the responsibility of the individual authors and neither the study nor this publication is associated with Aragen Life Sciences.
Open access
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuemmerle, A., Gossen, D., Marx, M.W. et al. A randomized, open-label two-period crossover pilot study to evaluate the relative bioavailability in the fed state of atovaquone-proguanil (Atoguanil™) versus atovaquone-proguanil hydrochloride (Malarone®) in healthy adult participants. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03245-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03245-x