Abstract
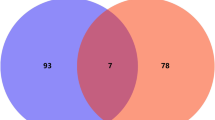
The present study aimed to investigate the anti-cancer mechanism of canagliflozin (CANA) and dapagliflozin (DAPA), sodium-glucose co-transporter-2 (SGLT2) inhibitors, using in silico and in vitro approaches. Network pharmacology was employed to predict the targets of the inhibitors and GO gene enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation conducted to explore the interacting pathways. Molecular docking and molecular dynamic (MD) simulation studies were performed to confirm the important targets and assess conformational stability. In vitro cytotoxicity assays, MIA-PaCa-2 and DU-145 cell lines CANA and DAPA was performed. Protein–protein interaction (PPI) network analysis indicated that CANA and DAPA exert anticancer effects through MAPK, mTOR, EGFR-KRAS-BRAF, FGFR, and PI3KA pathways. KEGG analysis revealed that these inhibitors could be used in the treatment of various cancers, including breast, prostate, pancreatic, chronic myeloid leukemia, thyroid, small cell lung, gastric, and bladder cancer. Docking results showed highest affinity for MAPK1 for CANA (− 9.60 kcal/mol) and DAPA (− 9.58 kcal/mol). MD simulation results showed that RMSD values for the MAPK1-compound exhibit remarkable stability over a timeframe of 100 ns. In cytotoxicity assays using MIA-PaCa-2 and DU-145 cell lines, CANA demonstrated a potential antiproliferative effect on the pancreatic cell line MIA-PaCa-2 after 48 h of treatment at a concentration of 100 µg/ml. Furthermore, CANA arrested the cell cycle in the sub-G1 phase and induced late apoptosis and necrosis in MIA-PaCa-2 cell line. Based on these findings, CANA shows promise as a potential novel treatment strategy for pancreatic cancer.










Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Basak D, Gamez D, Deb S (2023) SGLT2 Inhibitors as potential anticancer agents. Biomedicines 11(7):1867. https://doi.org/10.3390/biomedicines11071867
Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T, Ćwiertnia M, Hajduga MB (2022) SGLT-2 inhibitors in cancer treatment-mechanisms of action and emerging new perspectives. Cancers (basel) 14(23):5811. https://doi.org/10.3390/cancers14235811
Furukawa T (2015) Impacts of activation of the mitogen-activated protein kinase pathway in pancreatic cancer. Front Oncol 5:23. https://doi.org/10.3389/fonc.2015.00023
Haase M, Fitze G (2016) HSP90AB1: Helping the good and the bad. Gene 575(2 Pt 1):171–186. https://doi.org/10.1016/j.gene.2015.08.063
Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G et al (2011) Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther 19(1):188–195. https://doi.org/10.1038/mt.2010.216
Kardile RA, Sarkate AP, Lokwani DK, Tiwari SV, Azad R, Thopate SR (2023) Design, synthesis, and biological evaluation of novel quinoline derivatives as small molecule mutant EGFR inhibitors targeting resistance in NSCLC: in vitro screening and ADME predictions. Eur J Med Chem 245(Pt1):114889. https://doi.org/10.1016/j.ejmech.2022.114889
Koes DR, Baumgartner MP, Camacho CJ (2013) Lessons learned in empirical scoring with Smina from the CSAR 2011 benchmarking exercise. J Chem Inf Model 53(8):1893–1904. https://doi.org/10.1021/ci300604z
Kopetz S (2007) Targeting SRC and epidermal growth factor receptor in colorectal cancer: rationale and progress into the clinic. J Gastrointest Cancer 1(4 Suppl 2):S37-41
Leem J, Jung W, Park HJ, Kim K (2022) A network pharmacology-based approach to explore mechanism of action of medicinal herbs for alopecia treatment. Sci Rep 12(1):2852. https://doi.org/10.1038/s41598-022-06811-6
Lokwani DK, Chavan SR, Sarkate AP, Natarajan PM, Umapathy VR, Jain SP (2023) Virtual screening of natural compounds as potential SARS-CoV-2 main protease inhibitors: a molecular docking and molecular dynamics simulation guided approach. Chem Proc 14(1):85. https://doi.org/10.3390/ecsoc-27-16049
Magalhaes LG, Ferreira LLG, Andricopulo AD (2018) Recent advances and perspectives in cancer drug design. An Acad Bras Cienc 90(1 Suppl 2):1233–1250. https://doi.org/10.1590/0001-3765201820170823
More SA, Patil AS, Sakle NS, Mokale SN (2021) Network analysis and molecular mapping for SARS-CoV-2 to reveal drug targets and repurposing of clinically developed drugs. Virology 555:10–18. https://doi.org/10.1016/j.virol.2020.12.006
Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T (2016) Network pharmacology of cancer: from understanding of complex interactomes to design of multi-target specific therapeutics from nature. Pharmacol Res 111:290–302. https://doi.org/10.1016/j.phrs.2016.06.018
Rasul HO, Aziz BK, Ghafour DD, Kivrak A (2021) In silico molecular docking and dynamic simulation of eugenol compounds against breast cancer. J Mol Model 28(1):17. https://doi.org/10.1007/s00894-021-05010-w
Rasul HO, Aziz BK, Ghafour DD, Kivrak A (2023) Discovery of potential mTOR inhibitors from Cichorium intybus to find new candidate drugs targeting the pathological protein related to the breast cancer: an integrated computational approach. Mol Divers 27(3):1141–1162. https://doi.org/10.1007/s11030-022-10475-9
Ren D, Sun Y, Zhang D, Li D, Liu Z, Jin X, Wu H (2021) SGLT2 promotes pancreatic cancer progression by activating the Hippo signaling pathway via the hnRNPK-YAP1 axis. Cancer Lett 519:277–288. https://doi.org/10.1016/j.canlet.2021.07.035
Sakle NS, More SA, Mokale SN (2020) A network pharmacology-based approach to explore potential targets of Caesalpinia pulcherima: an updated prototype in drug discovery. Sci Rep 10:17217. https://doi.org/10.1038/s41598-020-74251-1
Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N et al (2015) Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA 112(30):E4111–E4119. https://doi.org/10.1073/pnas.1511698112
Shorning BY, Dass MS, Smalley MJ, Pearson HB (2020) The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci 21(12):4507. https://doi.org/10.3390/ijms21124507
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Sousa da Silva AW, Vranken WF (2012) ACPYPE - AnteChamber PYthon Parser InterfacE. BMC Res Notes 5:367. https://doi.org/10.1186/1756-0500-5-367
Stanciu S, Ionita-Radu F, Stefani C, Miricescu D, Stanescu-Spinu II, Greabu M, Ripszky Totan A, Jinga M (2022) Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer: from molecular to clinical aspects. Int J Mol Sci 23(17):10132. https://doi.org/10.3390/ijms231710132
Talevi A, Bellera CL (2020) Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov 15(4):397–401. https://doi.org/10.1080/17460441.2020.1704729
Thorat NM, Khodade VS, Ingale AP, Lokwani DK, Sarkate AP, Thopate SR (2023) Molecular docking studies and application of 6-(1-Arylmethanamino)-2-phenyl-4 H -chromen-4-ones as potent antibacterial Agents. Polycycl Aromat Compd 43(10):8653–8666. https://doi.org/10.1080/10406638.2022.2150238
Tiwari SV, Sarkate AP, Lokwani DK, Pansare DN, Gattani SG, Sheaikh SS et al (2022) Explorations of novel pyridine-pyrimidine hybrid phosphonate derivatives as aurora kinase inhibitors. Bioorganic Med Chem Lett 67:128747. https://doi.org/10.1016/j.bmcl.2022.128747
Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E (2021) Gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Compu 17(10):6281–6291. https://doi.org/10.1021/acs.jctc.1c00645
Wang Y, Yang L, Mao L, Zhang L, Zhu Y, Xu Y et al (2022) SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int 22(1):74. https://doi.org/10.1186/s12935-022-02496-z
Waters AM, Der CJ (2018) KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med 8(9):a031435. https://doi.org/10.1101/cshperspect.a031435
Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (basel) 9(5):52. https://doi.org/10.3390/cancers9050052
Zhou J, Zhu J, Yu SJ, Ma HL, Chen J, Ding XF et al (2020) Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother 132:110821. https://doi.org/10.1016/j.biopha.2020.110821
Acknowledgements
The authors are thankful to the Management and Principal of Y. B. Chavan College of Pharmacy, Aurangabad (MS), India, for providing the facilities and infrastructure to carry out the research work.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
The authors of this study, Prasanna Mohite, Deepak Lokwani, and Nikhil S. Sakle, made equal contributions to the research and manuscript preparation. Prasanna Mohite is responsible for designing the experiments and writing the manuscript. Deepak Lokwani conducted the molecular docking and molecular dynamics simulation study, while Nikhil S. Sakle performed the network pharmacology analysis, provided guidance and supervision throughout the research work, and edited the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohite, P., Lokwani, D.K. & Sakle, N.S. Exploring the therapeutic potential of SGLT2 inhibitors in cancer treatment: integrating in silico and in vitro investigations. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03021-x