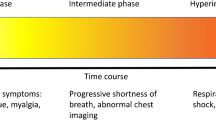
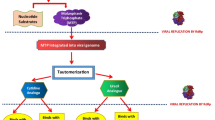
Abstract
Remdesivir (RDV) is the mainstay antiviral therapy for moderate to severe COVID-19. Although remdesivir was the first drug approved for COVID-19, information about its efficacy and safety profile is limited in a significant segment of the population, such as people with underlying diseases, the elderly, children, and pregnant and lactating women. The efficacy and safety profile of RDV in disease progression, renal impairment, liver impairment, immunosuppression, geriatrics, pediatrics, pregnancy, and breastfeeding in COVID-19 patients was evaluated. The databases searched included Embase, Scopus, and PubMed. Only English language studies enrolling specific subpopulations with COVID-19 and treated with RDV were included. Thirty-nine clinical trials, cohorts, cross-sectional studies, and case series/reports were included. Most supported the benefits of RDV therapy for COVID-19 patients, such as lessening the duration of hospitalization, alleviating respiratory complications, and reducing mortality. Adverse effects of RDV, including liver and kidney impairment, were, for the most part, moderate to mild, supporting the safety profile of RDV therapy. RDV therapy was well tolerated, no new safety signals were detected, and liver function test abnormalities were the most common adverse events. Moreover, RDV, for the most part, was effective in managing the complications of COVID-19 and reducing mortality in these patients, except for patients with kidney impairment. Future studies, including RCTs, should include these subpopulations of patients to avoid delays associated with receiving proper medication through compassionate use programs.

Similar content being viewed by others
Data availability
The datasets/information used for this study are available from the corresponding author upon reasonable request.
References
(2022) Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. The Lancet 399: 1941–1953
Abedi F, Rezaee R, Karimi G (2020) Plausibility of therapeutic effects of Rho kinase inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19). Pharmacol Res 156:104808
Abedi F, Rezaee R, Hayes AW, Nasiripour S, Karimi G (2021) MicroRNAs and SARS-CoV-2 life cycle, pathogenesis, and mutations: biomarkers or therapeutic agents? Cell Cycle 20:143–153
Ackley TW, McManus D, Topal JE, Cicali B, Shah S (2021) A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother 65:e02290–20
(ACOG) ACoOaG (2022) COVID-19 FAQs for obstetricians-gynecologists,obstetrics., https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics.
Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, Nigwekar S, Rhee EP, Sise ME (2020) Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol 31:1384–1386
Adarsh Bhimraj RLM, ** Amy Hirsch Shumaker, Lindsey Baden, Vincent Chi-Chung Cheng, Kathryn M. Edwards, Jason C. Gallagher, Rajesh T. Gandhi, William J. Muller, Mari M. Nakamura, John C. O’Horo, Robert W. Shafer, Shmuel Shoham, M. Hassan Murad,** Reem A. Mustafa,** Shahnaz Sultan,** Yngve Falck-Ytter** (2023) IDSA Guidelines on the treatment and management of patients with COVID-19. IDSA
Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, Spong CY (2020) Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open 3:e2029256
Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, Gubertini G, Coen M, Magni C, Castelli A, Borghi B, Colombo R, Giorgi R, Angeli E, Mileto D, Milazzo L, Vimercati S, Pellicciotta M, Corbellino M, Torre A, Rusconi S, Oreni L, Gismondo MR, Giacomelli A, Meroni L, Rizzardini G, Galli M (2020) Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res 158:104899
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-d, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC (2020a) Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 383:1813–1826
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC (2020b) Remdesivir for the treatment of Covid-19—final report. N Engl J Med 383:1813–1826
Biancalana E, Chiriacò M, Sciarrone P, Mengozzi A, Mechelli S, Taddei S, Solini A (2021) Remdesivir, Renal function and short-term clinical outcomes in elderly COVID-19 pneumonia patients: a single-centre study. Clin Interv Aging 16:1037–1046
Blann AD, Heitmar R (2022) SARS-CoV-2 and COVID-19: A Narrative Review. Br J Biomed Sci 79:10426
Boglione L, Dodaro V, Meli G, Rostagno R, Poletti F, Moglia R, Bianchi B, Esposito M, Borrè S (2022) Remdesivir treatment in hospitalized patients affected by COVID-19 pneumonia: a case-control study. J Med Virol 94:3653–3660
Burwick RM, Yawetz S, Stephenson KE, Collier AY, Sen P, Blackburn BG, Kojic EM, Hirshberg A, Suarez JF, Sobieszczyk ME, Marks KM, Mazur S, Big C, Manuel O, Morlin G, Rose SJ, Naqvi M, Goldfarb IT, DeZure A, Telep L, Tan SK, Zhao Y, Hahambis T, Hindman J, Chokkalingam AP, Carter C, Das M, Osinusi AO, Brainard DM, Varughese TA, Kovalenko O, Sims MD, Desai S, Swamy G, Sheffield JS, Zash R, Short WR (2021) Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis 73:e3996–e4004
Cacho J, Burgos E, Molina M, Villegas A, Pérez M, Cañas L, Taco O, Juega J, Lauzurica R (2022) Remdesivir in kidney transplant patients with SARS-CoV-2 pneumonia. Nefrologia (engl Ed) 42:311–317
Chavda VP, Teli D, Balar PC, Vaghela D, Solanki HK, Vaishnav A, Vora L (2023) Potential anti-SARS-CoV-2 prodrugs activated by phosphorylation and their role in the aged population. Molecules 28:2332
Choe PG, Jeong SI, Kang CK, Yang L, Lee S, Cho JY, Han SS, Kim DK, Lee SM, Park WB, Oh MD, Kim NJ (2022) Exploration for the effect of renal function and renal replacement therapy on pharmacokinetics of remdesivir and GS-441524 in patients with COVID-19: a limited case series. Clin Transl Sci 15:732–740
Chow EJ, Maust B, Kazmier KM, Stokes C (2021) Sinus bradycardia in a pediatric patient treated with remdesivir for acute coronavirus disease 2019: a case report and a review of the literature. J Pediatric Infect Dis Soc 10:926–929
Colaneri M, Amarasinghe N, Rezzonico L, Pieri TC, Segalini E, Sambo M, Roda S, Meloni F, Gregorini M, Rampino T, Pelenghi S, Ricciardi A, Bruno R (2022) Early remdesivir to prevent severe COVID-19 in recipients of solid organ transplant: a real-life study from Northern Italy. Int J Infect Dis 121:157–160
Dande R, Qureshi A, Persaud K, Puri C, Zulfiqar S, Awasthi S (2021) Remdesivir in a pregnant patient with COVID-19 pneumonia. J Community Hosp Intern Med Perspect 11:103–106
Davis MR, Pham CU, Cies JJ (2021) Remdesivir and GS-441524 plasma concentrations in patients with end-stage renal disease on haemodialysis. J Antimicrob Chemother 76:822–825
Eid J, Abdelwahab M, Colburn N, Day S, Cackovic M, Rood KM, Costantine MM (2022) Early administration of remdesivir and intensive care unit admission in hospitalized pregnant individuals with coronavirus disease 2019 (COVID-19). Obstet Gynecol 139:619–621
Elens L, Langman LJ, Hesselink DA, Bergan S, Moes D, Molinaro M, Venkataramanan R, Lemaitre F (2020) Pharmacologic treatment of transplant recipients infected with SARS-CoV-2: considerations regarding therapeutic drug monitoring and drug-drug interactions. Ther Drug Monit 42:360–368
Fesu D, Bohacs A, Hidvegi E, Matics Z, Polivka L, Horvath P, Czaller I, Sutto Z, Eszes N, Vincze K, Muller V (2022) Remdesivir in solid organ recipients for COVID-19 pneumonia. Transplant Proc 54:2567–2569
Fintzi J, Bonnett T, Sweeney DA, Huprikar NA, Ganesan A, Frank MG, McLellan SLF, Dodd LE, Tebas P, Mehta AK (2022) Deconstructing the treatment effect of remdesivir in the adaptive coronavirus disease 2019 (COVID-19) treatment trial-1: implications for critical care resource utilization. Clin Infect Dis 74:2209–2217
Frauenfelder C, Brierley J, Whittaker E, Perucca G, Bamford A (2020) Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics 146:e20201701
Garcia-Vidal C, Meira F, Cózar-Llistó A, Dueñas G, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Cardozo C, Hernandez-Meneses M, Alonso-Navarro R, Rico V, Agüero D, Bodro M, Morata L, Jordan C, Lopera C, Ambrosioni J, Segui F, Grafia N, Castro P, García F, Mensa J, Martínez JA, Sanjuan G, Soriano A (2021) Real-life use of remdesivir in hospitalized patients with COVID-19. Rev Esp Quimioter 34:136–140
Goldman DL, Aldrich ML, Hagmann SHF, Bamford A, Camacho-Gonzalez A, Lapadula G, Lee P, Bonfanti P, Carter CC, Zhao Y, Telep L, Pikora C, Naik S, Marshall N, Katsarolis I, Das M, DeZure A, Desai P, Cao H, Chokkalingam AP, Osinusi A, Brainard DM, Méndez-Echevarría A (2021) Compassionate use of remdesivir in children with severe COVID-19. Pediatrics 147:e2020047803
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA (2022) Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 386:305–315
Gutierrez R, Mendez-Figueroa H, Biebighauser JG, Bhalwal A, Pineles BL, Chauhan SP (2022) Remdesivir use in pregnancy during the SARS-CoV-2 pandemic. J Matern Fetal Neonatal Med 35:9445–9451
Habeeb E, Gabardi S, Townsend K, Kim M (2023) Potential effects of remdesivir on tacrolimus exposure in transplant recipients with COVID-19 infection. Kidney Int Rep 8:1315–1322
Health NIo (2023) Coronavirus disease 2019 (COVID-19) treatment guidelines
Herz-Roiphe R, Kim AY, Kaimal AJ, Goldfarb IT (2022) Utilizing labour and delivery units for remdesivir infusion for high-risk pregnant and postpartum patients with mild-to-moderate disease during a COVID-19 surge. J Hosp Infect 129:38–40
Hopwood AJ, Jordan-Villegas A, Gutierrez LD, Cowart MC, Vega-Montalvo W, Cheung WL, McMahan MJ, Gomez MR, Laham FR (2021) Severe acute respiratory syndrome coronavirus-2 pneumonia in a newborn treated with remdesivir and coronavirus disease 2019 convalescent plasma. J Pediatric Infect Dis Soc 10:691–694
Igbinosa I, Miller S, Bianco K, Nelson J, Kappagoda S, Blackburn BG, Grant P, Subramanian A, Lyell DJ, El-Sayed YY, Aziz N (2020) Use of remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am J Obstet Gynecol 223:768–770
Jo YH, Hwang Y, Choi SH (2021) A case report for severe covid-19 in a 9-year-old child treated with remdesivir and dexamethasone. J Korean Med Sci 36:e203
Jorgensen SCJ, Davis MR, Lapinsky SE (2021) A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother 77:24–30
Kanai O, Fujita K, Nanba K, Esaka N, Hata H, Seta K, Yasoda A, Odagaki T, Mio T (2021) Safety of remdesivir for patients 80 years of age or older with coronavirus disease 2019 (COVID-19). Drugs Aging 38:1067–1074
La Tessa A, Motisi MA, Marseglia GL, Cardinale F, Licari A, Manti S, Tosca M, Del Giudice MM, De Filippo M, Galli L, Chiappini E (2021) Use of remdesivir in children with COVID-19 infection: a quick narrative review. Acta Biomed 92:e2021524
LaCourse S, John-Stewart G, Adams Waldorf KM (2020) Importance of inclusion of pregnant and breastfeeding women in COVID-19 therapeutic trials. Clin Infect Dis 71:879–881
Lampejo T (2021) Remdesivir for the treatment of COVID-19 in pregnancy. J Med Virol 93:4114–4119
Lapadula G, Bernasconi DP, Bellani G, Soria A, Rona R, Bombino M, Avalli L, Rondelli E, Cortinovis B, Colombo E, Valsecchi MG, Migliorino GM, Bonfanti P, Foti G, Group R-RS (2020b) Remdesivir use in patients requiring mechanical ventilation due to COVID-19. Open Forum Infectious Diseases 7
Lapadula G, Bernasconi DP, Bellani G, Soria A, Rona R, Bombino M, Avalli L, Rondelli E, Cortinovis B, Colombo E, Valsecchi MG, Migliorino GM, Bonfanti P, Foti G (2020a) Remdesivir use in patients requiring mechanical ventilation due to COVID-19. Open Forum Infect Dis 7: ofaa481
Lee S, Santarelli A, Caine K, Schritter S, Dietrich T, Ashurst J (2020) Remdesivir for the treatment of severe COVID-19: a community hospital’s experience. J Am Osteopath Assoc 120:926–933
Lee H, Choi S, Park JY, Jo DS, Choi UY, Lee H, Jung YT, Chung IH, Choe YJ, Kim JY, Park YJ, Choi EH (2022) Analysis of critical COVID-19 cases among children in Korea. J Korean Med Sci 37:e13
Levien TL, Baker DE (2023) Remdesivir. Hosp Pharm 58:420–430
Liu E, Smyth RL, Li Q, Qaseem A, Florez ID, Mathew JL, Amer YS, Estill J, Lu Q, Fu Z, Lu X, Chan ES, Schwarze J, Wong GW, Fukuoka T, Ahn HS, Lee MS, Nurdiati D, Cao B, Tu W, Qian Y, Zhao S, Dong X, Luo X, Chen Z, Li G, Zhang X, Zhao X, Xu H, Xu F, Shi Y, Zhao R, Zhao Y, Lei J, Zheng X, Wang M, Yang S, Feng X, Wu L, He Z, Liu S, Wang Q, Song Y, Luo Z, Zhou Q, Guyatt G, Chen Y, Li Q (2022) Guidelines for the prevention and management of children and adolescents with COVID-19. Eur J Pediatr 181:4019–4037
Liu XI, Dallmann A, Brooks K, Best BM, Clarke DF, Mirochnick M, van den Anker JN, Capparelli EV, Momper JD (2023) Physiologically-based pharmacokinetic modeling of remdesivir and its metabolites in pregnant women with COVID-19. CPT Pharmacometrics Syst Pharmacol 12:148–153
López V, Vázquez T, Alonso-Titos J, Cabello M, Alonso A, Beneyto I, Crespo M, Díaz-Corte C, Franco A, González-Roncero F, Gutiérrez E, Guirado L, Jiménez C, Jironda C, Lauzurica R, Llorente S, Mazuecos A, Paul J, Rodríguez-Benot A, Ruiz JC, Sánchez-Fructuoso A, Sola E, Torregrosa V, Zárraga S, Hernández D (2020) Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients. Nefrologia (engl Ed) 40:265–271
Maldarelli GA, Savage M, Mazur S, Oxford-Horrey C, Salvatore M, Marks KM (2020) Remdesivir treatment for severe COVID-19 in third-trimester pregnancy: case report and management discussion. Open Forum Infect Dis 7: ofaa345
Manabe S, Mizuno S, Jinda T, Kasai M (2022) Safety of remdesivir in 20 children with COVID-19—case series. Biol Pharm Bull 45:1853–1856
Méndez-Echevarría A, Pérez-Martínez A, Gonzalez Del Valle L, Ara MF, Melendo S, Ruiz de Valbuena M, Vazquez-Martinez JL, Morales-Martínez A, Remesal A, Sándor-Bajusz KA, Cabañas F, Calvo C (2021) Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr 180:1317–1322
Meshram HS, Kute VB, Patel H, Banerjee S, Navadiya V, Desai S, Rizvi SJ, Mishra V, Chauhan S (2021) Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: a retrospective cohort from a developing nation. Transpl Infect Dis 23:e13629
Molaei E, Molaei A, Hayes AW, Karimi G (2021) Resolvin D1, therapeutic target in acute respiratory distress syndrome. Eur J Pharmacol 911:174527
Naqvi M, Zakowski P, Glucksman L, Smithson S, Burwick RM (2020) Tocilizumab and remdesivir in a pregnant patient with coronavirus disease 2019 (COVID-19). Obstet Gynecol 136:1025–1029
Orf K, Rogosic S, Dexter D, Ancliff P, Badle S, Brierley J, Cheng D, Dalton C, Dixon G, Du Pré P, Grandjean L, Ghorashian S, Mittal P, O’Connor D, Pavasovic V, Rao A, Samarasinghe S, Vora A, Bamford A, Bartram J (2020) Remdesivir during induction chemotherapy for newly diagnosed paediatric acute lymphoblastic leukaemia with concomitant SARS-CoV-2 infection. Br J Haematol 190:e274–e276
Pasquini Z, Montalti R, Temperoni C, Canovari B, Mancini M, Tempesta M, Pimpini D, Zallocco N, Barchiesi F (2020) Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J Antimicrob Chemother 75:3359–3365
Peters BJ, Rabinstein AA, DuBrock HM (2021) Use of remdesivir in myasthenia gravis and COVID-19. Pharmacotherapy 41:546–550
Sabers AJ, Williams AL, Farley TM (2020) Use of remdesivir in the presence of elevated LFTs for the treatment of severe COVID-19 infection. BMJ Case Rep 13
Saikia B, Tang J, Robinson S, Nichani S, Lawman KB, Katre M, Bandi S (2021) Neonates With SARS-CoV-2 infection and pulmonary disease safely treated with remdesivir. Pediatr Infect Dis J 40:e194–e196
Sarhan MA, Casalino M, Paopongsawan P, Gryn D, Kulkarni T, Bitnun A, Gauda EB (2022) SARS-CoV-2 associated respiratory failure in a preterm infant and the outcome after remdesivir treatment. Pediatr Infect Dis J 41:e233–e234
Seethapathy R, Zhao S, Long JD, Strohbehn IA, Sise ME (2022) A propensity score-matched observational study of remdesivir in patients with COVID-19 and severe kidney disease. Kidney360 3: 269–278
Shakir A, Bhasin N, Swami R, Mehta K, Sinha S, Ali SS, Bansode J (2021) Renal and hepatic outcomes after remdesivir therapy in coronavirus disease-2019-positive patients with renal dysfunction at baseline or after starting therapy. Saudi J Kidney Dis Transpl 32:1034–1042
Silva NAO, Zara A, Figueras A, Melo DO (2021) Potential kidney damage associated with the use of remdesivir for COVID-19: analysis of a pharmacovigilance database. Cad Saude Publica 37:e00077721
Sörgel F, Malin JJ, Hagmann H, Kinzig M, Bilal M, Eichenauer DA, Scherf-Clavel O, Simonis A, El Tabei L, Fuhr U, Rybniker J (2021) Pharmacokinetics of remdesivir in a COVID-19 patient with end-stage renal disease on intermittent haemodialysis. J Antimicrob Chemother 76:825–827
Thakare S, Gandhi C, Modi T, Bose S, Deb S, Saxena N, Katyal A, Patil A, Patil S, Pajai A, Bajpai D, Jamale T (2021) Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep 6:206–210
Thiede JM, Gress AR, Libby SD, Ronayne CE, Matchett WE, Noren B, Billings JL, Menachery VD, Langlois RA, Kline S, Bold TD (2021) Immune profiling to determine early disease trajectories associated with coronavirus disease 2019 mortality rate: a substudy from the ACTT-1 trial. J Infect Dis 223:1339–1344
Umemura T, Nishikawa K, Mutoh Y, Sasano H, Kozaki K, Yamada T, Ichihara T (2021) Usage experience of remdesivir for SARS-CoV-2 infection in a patient with chronic cirrhosis of Child-Pugh class C. J Antimicrob Chemother 76:1947–1948
Wada YS, Saito J, Hashii Y, Kishi T, Kobayashi M, Kamiya T, Mizuno K (2022) Remdesivir and human milk: a case study. J Hum Lact 38:248–251
Waddankeri S, Hesarur SS, Hiremath SS, Sangamesh A, Ar S, Raikod BP, Kinagi S, Biradar S, Belli B, Melkundi S, Singh G (2021) Clinical efficacy and safety of remdesivir among hospitalised adult patients with RT PCR confirmed COVID 19 requiring ICU care in Kalyana Karnataka. J Assoc Physicians India 69:11–12
Wang S, Huynh C, Islam S, Malone B, Masani N, Joseph D (2022a) Assessment of safety of remdesivir in Covid - 19 patients with estimated glomerular filtration rate (eGFR) < 30 ml/min per 1.73 m^2. J Intensive Care Med 37:764–768
Wang Z, Zhao S, Tang Y, Wang Z, Shi Q, Dang X, Gan L, Peng S, Li W, Zhou Q, Li Q, Mafiana JJ, Cortés RG, Luo Z, Liu E, Chen Y (2022b) Potentially effective drugs for the treatment of COVID-19 or MIS-C in children: a systematic review. Eur J Pediatr 181:2135–2146
Acknowledgements
The authors thank Mashhad University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
EM and AM performed the literature searches and data extraction and drafted parts of the manuscript. AWH revised the manuscript for critical intellectual content. GK conceptualized the work, supervised the project, and revised the manuscript for critical intellectual content. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable in this study.
Consent for publication
All the authors have read and agreed to the final copy of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molaei, E., Molaei, A., Hayes, A.W. et al. Remdesivir: treatment of COVID-19 in special populations. Naunyn-Schmiedeberg's Arch Pharmacol 397, 3829–3855 (2024). https://doi.org/10.1007/s00210-023-02927-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02927-2