Abstract
Coronavirus disease 2019 (COVID-19) has a wide-ranging spectrum of clinical symptoms, from asymptomatic/mild to severe. Recent research indicates that, among several factors, a low vitamin D level is a modifiable risk factor for COVID-19 patients. This study aims to evaluate the effect of vitamin D on hospital and laboratory outcomes of patients with COVID-19.
Five databases (PubMed, Embase, Scopus, Web of Science, and Cochrane Library) and clinicaltrials.gov were searched until July 2022, using relevant keywords/Mesh terms. Only randomized clinical trials (RCTs) that addressed the topic were included. The Cochrane tool was used to assess the studies’ risk of bias, and the data were analyzed using the review manager (RevMan 5.4).
We included nine RCTs with 1586 confirmed COVID-19 patients. Vitamin D group showed a significant reduction of intensive care unit (ICU) admission (risk ratio = 0.59, 95% confidence interval (CI) [0.41, 0.84], P = 0.003), and higher change in vitamin D level (standardized mean difference = 2.27, 95% CI [2.08, 2.47], P < 0.00001) compared to the control group. Other studied hospital and laboratory outcomes showed non-significant difference between vitamin D and the control group (P ≥ 0.05).
In conclusion, vitamin D reduced the risk of ICU admission and showed superiority in changing vitamin D level compared to the control group. However, other outcomes showed no difference between the two groups. More RCTs are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) is generated by the novel beta coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARSCoV2). The disease already had spread across the globe and leading the World Health Organization to declare it a pandemic. Since then, more than 508 million proven cases and 6 million mortalities have been reported through April 26, 2022 (Dong et al. 2020; Hu et al. 2021a; Cannata-Andía et al. 2022). COVID-19 has a wide spectrum of clinical symptoms, from asymptomatic or milder symptoms with fever, tiredness, and dry cough to severe and critical symptoms with dyspnea, intensive care unit (ICU) admission, acute respiratory distress syndrome, and multiorgan damage. Immunodeficiency, black ethnicity, older age, chronic kidney disease, obesity, and chronic metabolic disorders are risks related to COVID-19 severity (Xie et al. 2020; Hu et al. 2021b; Olumade and Uzairue 2021; Zhang et al. 2021; Guan et al. 2020).
Vitamin D deficiency was linked to the severity of viral diseases like influenza (Watkins et al. 2015). Recent research indicates that, among several factors, a low vitamin D level is a risk factor that can be modified for COVID-19 patients (Borna et al. 2022; Ilie et al. 2020; Grant et al. 2020; Meltzer et al. 2020). Vitamin D is known to have an antiinflammatory effect, modulate innate and adaptive immunological responses, and enhance the volume of antimicrobial proteins (AlSafar et al. 2021; Pinheiro et al. 2021; Malek Mahdavi 2020; Gois et al. 2017). According to new evidence, it possibly mitigates SARS-CoV-2 expression of the gene and reduces infection by binding to its receptor (Brito et al. 2021b; Glinsky 2020). However, there is no conclusive proof of vitamin D’s preventative and therapeutic significance in COVID-19 (Brito et al. 2021a).
Despite vaccine releases, considerable attention has been devoted to further preventive strategies, like vitamin D supplementation. Some studies showed the effectiveness of vitamin D in COVID-19, and they recommended it as a possible way of improving immune responses to COVID-19 vaccination (AlSafar et al. 2021; Graham 2020; Pinheiro et al. 2021; Malek Mahdavi 2020; Velikova et al. 2021). Also, some observational studies linked a lower vitamin D level to COVID-19 predisposition, morbidity, and mortality consequences (Angelidi et al. 2021; Bychinin et al. 2021; Campi et al. 2021; Infante et al. 2022; Orchard et al. 2021). However, there is no definite evidence of vitamin D supplementation’s beneficial and protective use in COVID-19 (Mercola et al. 2020; Petrelli et al. 2021; Fernandes et al. 2022; Rastogi et al. 2020; Tentolouris et al. 2022; Varikasuvu et al. 2022b; Cannata-Andía et al. 2022; Murai et al. 2021a; Soliman et al. 2021; Elamir et al. 2022; Cui et al. 2022). Therefore, this study aims to assess the effect of vitamin D on hospital and laboratory outcomes of COVID-19 patients.
Materials and methods
We depended on the PRISMA-P statement and the guideline of the Cochrane handbook for systematic reviews during this systematic review and meta-analysis (Higgins et al. 2019; Page et al. 2021).
Searching databases and keywords
Clinicaltrials.gov registry and five databases (Embase, Web of Science, PubMed, Scopus, and Cochrane Library) were searched until July 2022. We used the following search terms: “COVID 19,” “SARS CoV 2 Infection,” “COVID-19,” “Coronavirus,” “SARS-CoV-2 Infection,” “2019-nCoV Disease,” “SARS,” “Severe Acute Respiratory Syndrome,” “COVID19,” “Vitamin D,” “Calciol,” “Vitamin D 3,” “Vitamin D3,” “Cholecalciferol,” “25 Hydroxyvitamin D3,” “Calcidiol,” “25 Hydroxycholecalciferol,” “Calcifediol,” “Dedrogyl,” “Hydropherol,” “Calderol”. The search was not limited to any time or language. The above electronic search was complemented with a manual search in the reference records of included studies.
Eligibility criteria and study selection
All RCTs (S) that reported on COVID-19 patients (P) who received vitamin D supplementation (any type) (I) and compared their hospital and laboratory outcomes (O) with similar patients who received no intervention/placebo (C). Two types of outcomes were of this review focus as the following:
-
Primary outcomes (hospital): The need for ICU admission, ventilation and oxygen therapy, the risk of death, and the length of hospital stay (days).
-
Secondary outcomes (laboratory): The level of C-reactive protein (mg/dL), interleukin-6 (pg/mL), vitamin D concentration, lactate dehydrogenase (LDH), calcium concentration, creatinine, d-dimer, neutrophil count, lymphocyte count, platelet count, and leucocytes (no./μL).
Studies of other designs were excluded, including case reports, case series, reviews, editorials, in vitro, postmortem, conference abstract, letters to the editor, and author opinion papers. Titles and abstracts of potentially included studies were screened to include relevant ones, and then the full-texts were reviewed thoroughly to confirm the eligibility to be finally included. Four independent authors conducted the previous two steps, but in cases of indecision, a supervisor was involved to confirm the decision.
Data extraction and risk of bias assessment
Three authors extracted the following baseline items from the included trials: (a) general data: study arms, sample size, sex, age, and body mass index (BMI) (kg/m2) of participants; (b) comorbidities outcomes: diabetes, chronic obstructive pulmonary disease, hypertension, cardiovascular disease, and asthma; and (c) common COVID-19 symptoms: fever, cough, weakness, and diarrhea. Another three reviewers extracted the following summary data from the included trials, including NCT, vitamin D administration, follow-up period, and study’s primary outcomes and main findings. Six authors extracted the outcomes mentioned above.
The quality of the RCTs was appraised independently by five co-authors using the Cochrane tool to assess the risk of bias reported in the Cochrane Handbook for Systematic Reviews (part 2, chapter 8.5), which categorized the evaluated studies into three categories: high, low, or unclear risk. Indecisions, if any, were resolved through discussion and consensus with six co-authors.
Statistical analysis
We conducted this meta-analysis using Review Manager Software 5.4. Continuous outcomes were pooled as mean difference (MD) and 95% confidence intervals (CIs). In case of different assessment tools/devices, the data were pooled as standardized mean difference (SMD). Dichotomous outcomes were pooled as risk ratio (RR) and 95% CI. We pooled the data under the fixed-effect model and tested the heterogeneity between pooled studies by X2 and I2 tests. Once the heterogeneity between studies was detected (P-value < 0.1 and I2 > 50%), a random-effect model was used. We tried to solve the heterogeneity by sensitivity analysis using the leave-one-out method. The data were considered statistically significant if P-value < 0.05. Since the number of the included studies (n = 9) is less than 10, the publication bias could not be evaluated, according to Egger et al. (1997).
Results
Literature search
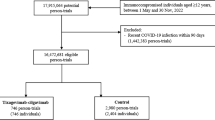
We retrieved 1244 records through an extended literature search on different search engines and excluded 571 papers by duplicate removal. The title and abstract screening excluded 641 articles. Thirty-two articles underwent full-text screening, and nine RCTs matched our criteria and entered all steps of meta-analysis to get the evidence (Cannata-Andía et al. 2022; Entrenas Castillo et al. 2020; Fernandes et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b; Rastogi et al. 2022; Mariani et al. 2022; Karonova et al. 2022; Soliman et al. 2021). See the PRISMA chart in Fig. 1.
Characteristics of included studies
We included all RCTs that studied the effect of vitamin D on hospital and laboratory outcomes of 1586 confirmed COVID-19 patients with a mean (SD) age of 56.41 (11.69). The intervention and control groups sample ranged from 16 to 274 participants, and males were more prominent than females. COVID-19 symptoms varied among patients, including fever, cough, general weakness, and diarrhea. Most patients received oral administration regarding vitamin D supplementation, but a single group received an intramuscular injection. Most participants suffered from comorbidities such as hypertension, diabetes, or asthma. Researchers specified the follow-up duration by either period ranged from 7 days to 4 months or an event such as intensive care unit admission, hospital discharge, or death. The summary and the baseline features of included RCTs are shown in Tables 1 and 2.
Risk of bias
The quality of the selected RCTs ranged from moderate to high quality. Eight and six studies were low-biased in random sequence generation and allocation concealment domains, respectively. Participants’ blinding occurred in four trials, while the blinding of outcome assessors occurred in five. Seven studies contained no attrition bias. Reporting bias domain was low-biased in all the included trials. Five studies were judged as high biased regarding other sources of bias domain. The risk of bias graph is presented in Fig. 2.
Analysis of the outcomes
Patients who required ICU admission
Five trials reported this outcome in 671 patients (Entrenas Castillo et al. 2020; Maghbooli et al. 2021; Murai et al. 2021b; Mariani et al. 2022; Karonova et al. 2022). The pooled data showed a significant lower incidence of patients who required ICU admission in vitamin D group compared with placebo group (RR = 0.59, 95% CI [0.41, 0.84], P = 0.003) and the pooled analysis was heterogeneous (P = 0.02, I2 = 66%) (Fig. 3A). We used random-effect model and sensitivity analysis by excluding Entrenas Castillo et al. (2020) trial to solve the heterogeneity (P = 0.54, I2 = 0%), and results become insignificant (RR = 0.8, 95% CI [0.54, 1.18], P = 0.26) (Fig. 3B).
Patients who required ventilation
Totally, 561 patients from three trials reported this outcome (Maghbooli et al. 2021; Murai et al. 2021b; Mariani et al. 2022). The overall estimate showed insignificant superiority of the vitamin D group over the placebo group (RR = 0.55, 95% CI [0.31, 1], P = 0.04), and the homogeneity was obvious among trials (P = 0.8, I2 = 0%) (Fig. 4).
Patients who required oxygen therapy
The pooled data of two studies with 216 participants (Maghbooli et al. 2021; Karonova et al. 2022) revealed insignificant variation between the comparison groups (RR = 0.94 95% CI [0.74, 1.18], P = 0.58). The pooled studies were homogeneous (P = 0.96, I2 = 0%) (Fig. 5).
Length of hospital stay (days)
Three trials reported this outcome in 867 patients (Cannata-Andía et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b). The intervention group showed insignificant superiority over the control group (MD = − 0.54, 95% CI [− 1.25, 0.18], P = 0.14), and the heterogeneity was detected (P = 0.03, I2 = 72%) (Fig. 6A). Heterogeneity was solved after excluding Cannata-Andía et al. (2022) trial (P = 0.55, I2 = 35%), and results become significant (MD = − 1.42, 95% CI [− 2.4, − 0.44], P = 0.005) (Fig. 6B).
Death
Death was reported by five trials involving 1160 patients (Cannata-Andía et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b; Mariani et al. 2022; Soliman et al. 2021). The overall estimate was non-significant (RR = 1.33, 95% CI [0.85, 2.06], P = 0.21), and the homogeneity between trials was observed (P = 0.75, I2 = 0%) (Fig. 7).
Change in interleukin-6 (pg/mL)
Three trials with 424 patients reported this outcome (Cannata-Andía et al. 2022; Fernandes et al. 2022; Karonova et al. 2022) and showed a non-significant overall effect size between groups (MD = − 1.54, 95% CI [− 7.74, 4.67], P = 0.63). The analysis was homogeneous (P = 0.57, I2 = 0%) (Suppl. Figure 1).
Change in C-reactive protein
Four trials reported this outcome in 690 participants (Cannata-Andía et al. 2022; Fernandes et al. 2022; Rastogi et al. 2022; Karonova et al. 2022), and the intervention group did not show significant results compared to the control group (SMD = − 0.08, 95% CI [− 0.23, 0.07], P = 0.29), and the results were homogeneous (P = 0.44, I2 = 0%) (Suppl. Figure 2).
Change in vitamin D
Four trials with 744 patients reported this outcome (Cannata-Andía et al. 2022; Fernandes et al. 2022; Rastogi et al. 2022; Karonova et al. 2022). The vitamin D group showed significant superiority over the placebo group (SMD = 2.27, 95% CI [2.08, 2.47], P < 0.00001), and the analysis was heterogeneous (P > 0.00001, I2 = 98%) (Suppl. Figure 3a). Under random-effect model the results were still significant (SMD = 2.62, 95% CI [0.95, 4.29], P = 0.002) and the heterogeneity could not be solved by sensitivity analysis (Suppl. Figure 3b).
Change in LDH (U/L)
This outcome was reported by two trials in 217 patients (Cannata-Andía et al. 2022; Maghbooli et al. 2021). The effect size showed insignificant change between the vitamin D and placebo groups (MD = 9.93, 95% CI [− 45.57, 65.44], P = 0.73), and the analysis was homogeneous (P = 0.63, I2 = 0%) (Suppl. Figure 4).
Change in serum calcium (mg/dL)
Three trials with 538 patients reported this outcome (Cannata-Andía et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b). The mean difference revealed no significant results (MD = 0.02, 95% CI [− 0.1, 0.15], P = 0.72), and the trials were homogeneous (P = 0.36, I2 = 3%) (Suppl. Figure 5).
Change in serum creatinine level (mg/dL)
The effect estimate of three trials with 577 patients (Cannata-Andía et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b) was not significant (MD = 0.02, 95% CI [− 0.04, 0.09], P = 0.44), and the data were heterogeneous (P = 0.08, I2 = 60%) (Suppl. Figure 6a). Heterogeneity best solved after Cannata-Andía et al. (2022) trial exclusion (P = 0.37, I2 = 0%), and results stayed insignificant (MD = − 0.06, 95% CI [− 0.17, 0.04], P = 0.23) (Suppl. Figure 6b).
Change in d-dimer
Two trials with 277 participants (Murai et al. 2021b; Rastogi et al. 2022) reported insignificant variation between the groups (SMD = − 0.11, 95% CI [− 0.34, 0.13], P = 0.37), and the pooled trials were homogeneous (P = 0.69, I2 = 0%) (Suppl. Figure 7).
Change in neutrophil count (× 103/mm3)
The overall results of three trials which included 445 participants (Maghbooli et al. 2021; Murai et al. 2021b; Karonova et al. 2022) showed insignificant results (MD = − 0.29, 95% CI [− 0.65, − 0.07], P = 0.11), and the heterogeneity between trials was observed (P = 0.07, I2 = 63%) (Suppl. Figure 8a). We used random-effect model and sensitivity analysis by excluding Karonova et al. (2022) trial to solve the heterogeneity (P = 0.3, I2 = 8%), and results were still insignificant (MD = − 0.35, 95% CI [− 0.83, 0.12], P = 0.14) (Suppl. Figure 8b).
Change in lymphocyte count (× 103/mm3)
Three trials with 445 patients (Maghbooli et al. 2021; Murai et al. 2021b; Karonova et al. 2022) showed insignificant variation between the study groups (MD = − 0.04, 95% CI [− 0.28, 0.2], P = 0.74), and the analysis was heterogeneous (P = 0.03, I2 = 73%) (Suppl. Figure 9a). We used random-effect model and sensitivity analysis by excluding Maghbooli et al. (2021) trial to solve the heterogeneity (P = 0.14, I2 = 55%), and results were still insignificant (MD = − 0.03, 95% CI [− 0.39, 0.33], P = 0.86) (Suppl. Figure 9b).
Change in platelet count (× 103/mm3)
The estimate of two trials with 340 patients (Maghbooli et al. 2021; Murai et al. 2021b) showed no significant favor of the intervention over the control group (MD = − 5.63, 95% CI [− 41.39, 30.12], P = 0.76), and the pooled analysis was homogeneous (P = 0.83, I2 = 0%) (Suppl. Figure 10).
Change in leucocytes (no./μL)
Three studies with 657 participants (Cannata-Andía et al. 2022; Fernandes et al. 2022; Maghbooli et al. 2021) reported this outcome and the results were non-significant (MD = − 0.19, 95% CI [− 0.8, 0.42], P = 0.55) (Suppl. Figure 11a). Heterogeneity between the groups was observed (P = 0.06, I2 = 64%), and solved after excluding Maghbooli et al. (2021) (P = 0.73, I2 = 0%). Under random-effect model, the results were still insignificant (MD = 0.16, 95% CI [− 0.52, 0.83], P = 0.65) (Suppl. Figure 11b).
Discussion
This systematic review and meta-analysis of nine RCTs aimed to find a definitive role of vitamin D on hospital and laboratory outcomes of COVID-19 patients. The analysis showed a significantly reduced risk of ICU admission. Also, vitamin D3 levels significantly affect its level positively. However, administration of vitamin D showed no significant difference compared to placebo regarding most hospital-related outcomes of the COVID-19 disease, including requiring ventilation, requiring oxygen therapy, death rate, and length of hospital stay. As for laboratory outcomes, a non-significant difference was also detected in the change in levels of interleukin-6, C-reactive protein, LDH, serum calcium, serum creatinine, d-dimer, neutrophil count, lymphocyte count, platelet count, and leucocytic count.
As for the ICU admission, our results showed a significant reduction in COVID-19 patients who received vitamin D. However, after solving the heterogeneity, the results turned non-significant. Our results were supported by another meta-analysis that concluded the positive effect of vitamin D on ICU admission; however, this study included observational studies, which may affect the results (Shah et al. 2021). Another meta-analysis of six studies suggested the influential role of vitamin D in ICU admission (Tentolouris et al. 2022). In another RCT, a significantly lower likelihood of ICU admission was maintained even after correcting for comorbidities such as hypertension and diabetes (Entrenas Castillo et al. 2020).
The previously mentioned results differ from Rawat et al., which excluded the retrospective study and found a non-significant effect on ICU admission (Rawat et al. 2021). The first multicenter, double-blind RCT in moderate-severe COVID-19 patients concluded that receiving a single high dosage of vitamin D3 (200,000 IU orally) did not lower the ICU admission, length of hospital stay, or rates of mechanical ventilation compared to peanut oil (Murai et al. 2021b). In another multicenter RCT on mild-moderate COVID-19 patients, insignificant changes in ICU or mortality events were observed even though the vitamin D arm had a considerably quicker recovery time to symptoms (even after controlling for age, gender, BMI, and d-dimer) (Sabico et al. 2021). The variations between the abovementioned studies may be due to the different comorbidities, the standard of care, severity of COVID-19, and vitamin D levels at the beginning of each trial. Regarding ventilation, previous studies reported inconsistent results with ours (Rawat et al. 2021; Bassatne et al. 2021; Murai et al. 2021a, b; Maghbooli et al. 2021). However, Maghbooli et al. concluded that vitamin D would benefit COVID-19 patients despite the insignificant results (Maghbooli et al. 2021).
Previous studies supported our results regarding death from COVID-19 and found that vitamin D did not reduce mortality (Tentolouris et al. 2021; Bassatne et al. 2021; Shah et al. 2021; Rawat et al. 2021; Cannata-Andía et al. 2022; Hernández et al. 2021; Murai et al. 2021b; Sabico et al. 2021; Beran et al. 2022). In contrast, Varikasuvu et al. reported that vitamin D significantly reduces mortality (Varikasuvu et al. 2022a). Other studies also reported a significant reduction in mortality favoring vitamin D over placebo (Nikniaz et al. 2021; Pal et al. 2022). Furthermore, a positive association between vitamin D insufficiency and the increased mortality from COVID-19 was detected, especially in the elderly (Pereira et al. 2022). This was explained by lower exposure to the sun, lower levels of 7-dehydrocholesterol in the skin, higher risk of severe COVID-19 due to comorbidities, and interference of vitamin D levels by the drugs used to treat these comorbidities (Adami et al. 2009; Pimenta et al. 2015; Grant et al. 2020; Jin et al. 2020). Also, Drame et al., in their systematic review, suggested an association between vitamin D deficiency and increased risk of COVID-19 positivity, unfavorable disease course, bad outcomes regarding mortality, disease severity, oxygen therapy requirements, and ventilation need (Dramé et al. 2021).
Elamir et al. reported that the intervention group did not affect the length of hospital stay and intubation need, which supports our results; however, they reported a significant reduction in oxygen therapy requirements, which is inconsistent with ours (Elamir et al. 2022). They explained this by the small number of participants in the trial but suggested a beneficial role of vitamin D on respiration (Elamir et al. 2022). A recent meta-analysis reported that vitamin D benefits both length of hospital stay and intubation requirements, which contrasts with our results (Beran et al. 2022). Another cohort analysis of the length of hospital stay and the death rate showed superiority in the highest serum calcidiol group (> 25 ng/mL) (Cannata-Andía et al. 2022). However, Bassatne et al. reported insignificant results (Bassatne et al. 2021). So, determination of any vitamin D deficiency in any patients is mandatory as the baseline vitamin D level would influence the benefits of its supplementation and the COVID-19 outcomes (Griffin et al. 2020).
Hypercalcemia was not observed in our included studies either in intervention or control groups, which means no difference between groups and proves the safety of vitamin D on the calcium level (Elamir et al. 2022; Rastogi et al. 2022). Previous research found a significant increase from baseline in vitamin D levels after vitamin D3 supplementation, consistent with our results (Fernandes et al. 2022; Rastogi et al. 2022; Murai et al. 2021b; Soliman et al. 2021). In similarity to our results, other studies reported insignificant results regarding d-dimer, CRP, IL-6, and LDH levels (Rawat et al. 2021; Rastogi et al. 2022; Fernandes et al. 2022; Maghbooli et al. 2021). It is known that COVID-19 raises inflammatory markers like d-dimer, fibrinogen, IL-6, and CRP, especially in severe cases, which are considered good indicators for severity and recovery of COVID-19 (Velavan and Meyer 2020).
Previous studies reported consistent results with ours regarding serum creatinine levels (Cannata-Andía et al. 2022; Maghbooli et al. 2021; Murai et al. 2021b). Furthermore, similar to our results regarding the change in the count of platelets, lymphocytes, and leucocytes, some researchers reported insignificant results, but others reported significant results regarding lymphocytic count (Maghbooli et al. 2021; Murai et al. 2021b). In response to inflammation such as COVID-19 events, leukocytes provide innate immunity, and lymphocytes provide adaptive immunity, so body defense occurs (Denman 1979).
Research proved that vitamin D exerts a biological effect in modulating the innate immune response, regulating the adaptive immune response, interacting with the renin‐angiotensin‐aldosterone system, protecting the endothelial functions, and yielding an antithrombotic action (Charoenngam et al. 2021; Griffin et al. 2020; Bilezikian et al. 2020; Arnold 2020; Malek Mahdavi 2020). These mechanisms reduce cytokine storm risk, enhance the immune response, and produce antiinflammatory, antiviral, and antimicrobial activities (Mercola et al. 2020; Teymoori-Rad et al. 2019; Pinheiro et al. 2021; Malek Mahdavi 2020; Gois et al. 2017). These protective functions of vitamin D were observed in patients with respiratory diseases (Jolliffe et al. 2021; Lips 2021; AlSafar et al. 2021) and patients who received the COVID-19 vaccine (Velikova et al. 2021; Chiu et al. 2021).
An acute illness such as COVID-19 reduces the circulation of vitamin D binding protein and interferes with the effective production of the body’s active form of vitamin D (Zehnder et al. 2001; Waldron et al. 2013). These phenomena may help explain the conflict between studies regarding the effectiveness of vitamin D on COVID-19.
Our study has several strengths which support the quality of the evidence. For example, we applied a comprehensive search strategy and literature search on different databases without language or time restrictions. We included only relevant RCTs that studied clinical and laboratory outcomes and excluded any other design. The included trials are considered low-biased regarding many quality assessment domains, which is supportive.
However, we found high heterogeneity between the included studies, such as different populations’ characteristics, including age, sex, race, body mass index, general status, the severity of COVID-19 symptoms, treatment protocol of the patients, and associated comorbidities. The regimens of vitamin D supplementation also varied across the studies regarding the form, the dose, the timing of administration, and the baseline levels of vitamin D. Patients received variable amounts of vitamin D, ranging from low to high doses and from single to daily doses. Previous research found that the daily doses of vitamin D prevent and treat certain diseases such as acute respiratory infections, rickets, and tuberculosis better than the intermittent doses (Griffin et al. 2021). Most of the studies included a low sample size, which also affected the quality of the evidence. During acute illness, vitamin D binding protein and albumin tend to decrease by the negative acute phase response, which affects vitamin D levels bound to them (Rhodes et al. 2021). Time of vitamin D administration also would impact its effect as most patients have received it after being infected and diagnosed with COVID-19.
Conclusions
Our study suggested that vitamin D supplementation benefits COVID‐19 patients by reducing ICU admission and increasing changes in vitamin D levels. However, it produces no difference in other outcomes compared to no vitamin D intake. The definite role of vitamin D on COVID‐19 outcomes strongly needs further well-conducted and high-quality research, especially after its known effect on the body’s immune system and defense mechanisms and the previously collected data on its benefits on certain respiratory diseases, including COVID-19.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adami S, Bertoldo F, Braga V, Fracassi E, Gatti D, Gandolini G, Minisola S, Battista Rini G (2009) 25-hydroxy vitamin D levels in healthy premenopausal women: association with bone turnover markers and bone mineral density. Bone 45:423–426
AlSafar H, Grant WB, Hijazi R, Uddin M, Alkaabi N, Tay G, Mahboub B, Al Anouti F (2021) COVID-19 disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients 13:1714–1714
Angelidi AM, Belanger MJ, Lorinsky MK, Karamanis D, Chamorro-Pareja N, Ognibene J, Palaiodimos L, Mantzoros CS (2021) Vitamin D status is associated with in-hospital mortality and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc 96:875–886
Arnold RH (2020) COVID-19 — does this disease kill due to imbalance of the renin angiotensin system (RAS) caused by genetic and gender differences in the response to viral ACE 2 attack? Heart Lung Circ 29:964–972
Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G (2021) The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metabolism 119:154753
Beran A, Mhanna M, Srour O, Ayesh H, Stewart JM, Hjouj M, Khokher W, Mhanna AS, Ghazaleh D, Khader Y, Sayeh W, Assaly R (2022) Clinical significance of micronutrient supplements in patients with coronavirus disease 2019: a comprehensive systematic review and meta-analysis. Clin Nutr ESPEN 48:167–177
Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, Madhavan MV, Nair N, Babalyan V, Hutchings N, Napoli N, Accili D, Binkley N, Landry DW, Giustina A (2020) Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol 183:R133-r147
Borna M, Woloshynowych M, Schiano-Phan R, Volpi EV, Usman M (2022) A correlational analysis of COVID-19 incidence and mortality and urban determinants of vitamin D status across the London boroughs. Sci Rep 12:11741
Brito DTM, Ribeiro LHC, Daltro C, Silva RB (2021a) The possible benefits of vitamin D in COVID-19. Nutrition 91–92:111356
Brito DTM, Ribeiro LHC, Daltro CH, d. C. & Silva, R. d. B. (2021b) The possible benefits of vitamin D in COVID-19. Nutrition 91–92:111356–111356
Bychinin MV, Klypa TV, Mandel IA, Andreichenko SA, Baklaushev VP, Yusubalieva GM, Kolyshkina NA, Troitsky AV (2021) Low circulating vitamin D in intensive care unit-admitted COVID-19 patients as a predictor of negative outcomes. J Nutr 151:2199–2205
Campi I, Gennari L, Merlotti D, Mingiano C, Frosali A, Giovanelli L, Torlasco C, Pengo MF, Heilbron F, Soranna D, Zambon A, Di Stefano M, Aresta C, Bonomi M, Cangiano B, Favero V, Fatti L, Perego GB, Chiodini I, Parati G, Persani L (2021) Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis 21:566–566
Cannata-Andía JB, Díaz-Sottolano A, Fernández P, Palomo-Antequera C, Herrero-Puente P, Mouzo R, Carrillo-López N, Panizo S, Ibañez GH, Cusumano CA, Ballarino C, Sánchez-Polo V, Pefaur-Penna J, Maderuelo-Riesco I, Calviño-Varela J, Gómez MD, Gómez-Alonso C, Cunningham J, Naves-Díaz M, Douthat W, Fernández-Martín JL (2022) A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med 20:83
Charoenngam N, Shirvani A, Holick MF (2021) Vitamin D and its potential benefit for the COVID-19 pandemic. Endocr Pract 27:484–493
Chiu SK, Tsai KW, Wu CC, Zheng CM, Yang CH, Hu WC, Hou YC, Lu KC, Chao YC (2021) Putative role of vitamin D for COVID-19 vaccination. Int J Mol Sci. 22(16):8988. https://doi.org/10.3390/ijms22168988
Cui X, Zhai Y, Wang S, Ding K, Yang Z, Tian Y, Huo T (2022) Effect of the COVID-19 pandemic on serum vitamin D levels in people under age 18 years: a systematic review and meta-analysis. Med Sci Monit 28:e935823
Denman AM (1979) Lymphocyte function and virus infections. J Clin Pathol Suppl (r Coll Pathol) 13:39–47
Dong E, Du H, Gardner L (2020) An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20:533–534
Dramé M, Cofais C, Hentzien M, Proye E, Coulibaly PS, Demoustier-Tampère D, Destailleur M-H, Lotin M, Cantagrit E, Cebille A, Desprez A, Blondiau F, Kanagaratnam L, Godaert L (2021) Relation between vitamin D and COVID-19 in aged people: a systematic review. Nutrients 13:1339
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Elamir YM, Amir H, Lim S, Rana YP, Lopez CG, Feliciano NV, Omar A, Grist WP, Via MA (2022) A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 154:116175
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM (2020) Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol 203:105751
Fernandes AL, Murai IH, Reis BZ, Sales LP, Santos MD, Pinto AJ, Goessler KF, Duran CSC, Silva CBR, Franco AS, Macedo MB, Dalmolin HHH, Baggio J, Balbi GGM, Antonangelo L, Caparbo VF, Gualano B, Pereira RMR (2022) Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am J Clin Nutr 115:790–798
Glinsky GV (2020) Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines 8:129–129
Gois PHF, Ferreira D, Olenski S, Seguro AC (2017) Vitamin D and infectious diseases: simple bystander or contributing factor? Nutrients 9:651
Graham BS (2020) Rapid COVID-19 vaccine development. Science 368:945–946
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP (2020) Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12(4):988. https://doi.org/10.3390/nu12040988
Griffin G, Hewison M, Hopkin J, Kenny R, Quinton R, Rhodes J, Subramanian S, Thickett D (2020) Vitamin D and COVID-19: evidence and recommendations for supplementation. R Soc Open Sci 7:201912
Griffin G, Hewison M, Hopkin J, Kenny RA, Quinton R, Rhodes J, Subramanian S, Thickett D (2021) Perspective: Vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: implications for COVID-19. Clin Med (lond) 21:e144–e149
Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ, Crespo J, Martínez-Taboada VM (2021) Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab 106:e1343–e1353
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons
Hu B, Guo H, Zhou P, Shi Z-L (2021a) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154
Ilie PC, Stefanescu S, Smith L (2020) The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 32:1195–1198
Infante M, Buoso A, Pieri M, Lupisella S, Nuccetelli M, Bernardini S, Fabbri A, Iannetta M, Andreoni M, Colizzi V, Morello M (2022) Low vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: an Italian retrospective study. J Am Nutr Assoc 41:250–265
Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH (2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 7:4
Jolliffe DA, Camargo CA Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, Bergman P, Bischoff-Ferrari HA, Borzutzky A, Damsgaard CT, Dubnov-Raz G, Esposito S, Gilham C, Ginde AA, Golan-Tripto I, Goodall EC, Grant CC, Griffiths CJ, Hibbs AM, Janssens W, Khadilkar AV, Laaksi I, Lee MT, Loeb M, Maguire JL, Majak P, Mauger DT, Manaseki-Holland S, Murdoch DR, Nakashima A, Neale RE, Pham H, Rake C, Rees JR, Rosendahl J, Scragg R, Shah D, Shimizu Y, Simpson-Yap S, Trilok-Kumar G, Urashima M, Martineau AR (2021) Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol 9:276–292
Karonova TL, Golovatyuk KA, Kudryavtsev IV, Chernikova AT, Mikhaylova AA, Aquino AD, Lagutina DI, Zaikova EK, Kalinina OV, Golovkin AS, Grant WB, Shlyakhto EV (2022) Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID-19 patients: a randomized, open-label, single-center study. Nutrients 14(13):2602. https://doi.org/10.3390/nu14132602
Lips P (2021) Vitamin D to prevent acute respiratory infections. Lancet Diabetes Endocrinol 9:249–251
Maghbooli Z, Sahraian MA, Jamalimoghadamsiahkali S, Asadi A, Zarei A, Zendehdel A, Varzandi T, Mohammadnabi S, Alijani N, Karimi M, Shirvani A, Holick MF (2021) Treatment with 25-hydroxyvitamin D(3) (calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: a pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr Pract 27:1242–1251
Malek Mahdavi A (2020) A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: implications for a potential treatment for COVID-19. Rev Med Virol 30:e2119
Mariani J, Antonietti L, Tajer C, Ferder L, Inserra F, Sanchez Cunto M, Brosio D, Ross F, Zylberman M, López DE, Luna Hisano C, Maristany Batisda S, Pace G, Salvatore A, Hogrefe JF, Turela M, Gaido A, Rodera B, Banega E, Iglesias ME, Rzepeski M, Gomez Portillo JM, Bertelli M, Vilela A, Heffner L, Annetta VL, Moracho L, Carmona M, Melito G, Martínez MJ, Luna G, Vensentini N, Manucha W (2022) High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: multicentre randomized controlled clinical trial. PLoS ONE 17:e0267918
Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J (2020) Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 3:e2019722
Mercola J, Grant WB, Wagner CL (2020) Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 12(11):3361. https://doi.org/10.3390/nu12113361
Murai IH, Fernandes AL, Antonangelo L, Gualano B, Pereira RMR (2021a) Effect of a single high-dose vitamin D3 on the length of hospital stay of severely 25-hydroxyvitamin D-deficient patients with COVID-19. Clinics (sao Paulo) 76:e3549
Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, Silva CBR, Franco AS, Macedo MB, Dalmolin HHH, Baggio J, Balbi GGM, Reis BZ, Antonangelo L, Caparbo VF, Gualano B, Pereira RMR (2021b) Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 325:1053–1060
Nikniaz L, Akbarzadeh MA, Hosseinifard H, Hosseini M-S (2021) The impact of vitamin D supplementation on mortality rate and clinical outcomes of COVID-19 patients: a systematic review and meta-analysis. Pharm Sci 27:S1–S12
Olumade TJ, Uzairue LI (2021) Clinical characteristics of 4499 COVID-19 patients in Africa: a meta-analysis. J Med Virol 93:3055–3061
Orchard L, Baldry M, Nasim-Mohi M, Monck C, Saeed K, Grocott MPW, Ahilanandan D (2021) Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med 59:1155–1163
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A (2022) Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest 45:53–68
Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J (2022) Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr 62:1308–1316
Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A (2021) Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol 211:105883–105883
Pimenta FB, Pinho L, Silveira MF, Botelho AC (2015) Factors associated with chronic diseases among the elderly receiving treatment under the Family Health Strategy. Cien Saude Colet 20:2489–2498
Pinheiro MM, Fabbri A, Infante M (2021) Cytokine storm modulation in COVID-19: a proposed role for vitamin D and DPP-4 inhibitor combination therapy (VIDPP-4i). Immunotherapy 13:753–765
Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD, Malhotra P (2022) Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J 8(1156):87–90. https://doi.org/10.1136/postgradmedj-2020-139065
Rastogi, A, Bhansali, A, Khare, N, Suri, V, Yaddanapudi, N, Sachdeva, N, Puri, GD, Malhotra, P (2020) Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J, postgradmedj-139065
Rawat D, Roy A, Maitra S, Shankar V, Khanna P, Baidya DK (2021) Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr 15:102189
Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA (2021) Perspective: Vitamin D deficiency and COVID-19 severity — plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 289:97–115
Sabico, S, Enani, MA, Sheshah, E, Aljohani, NJ, Aldisi, DA, Alotaibi, NH, Alshingetti, N, Alomar, SY, Alnaami, AM, Amer, OE, Hussain, SD, Al-Daghri, NM (2021) Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate COVID-19: a randomized clinical trial. Nutrients. 13(7):2170. https://doi.org/10.3390/nu13072170
Shah K, Saxena D, Mavalankar D (2021) Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM 114:175–181
Soliman, AR, Abdelaziz, TS, Fathy, A (2021) Impact of vitamin D therapy on the progress COVID-19: six weeks follow-up study of vitamin D deficient elderly diabetes patients. Proc Singap Healthc 31:20101058211041405. https://doi.org/10.1177/20101058211041405
Tentolouris N, Samakidou G, Eleftheriadou I, Tentolouris A, Jude EB (2022) The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev 38(4):e3517. https://doi.org/10.1002/dmrr.3517
Tentolouris, N, Samakidou, G, Eleftheriadou, I, Tentolouris, A, Jude, EB (2021) The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev, e3517
Teymoori-Rad M, Shokri F, Salimi V, Marashi SM (2019) The interplay between vitamin D and viral infections. Rev Med Virol 29:e2032
Varikasuvu SR, Thangappazham B, Vykunta A, Duggina P, Manne M, Raj H, Aloori S (2022b) COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther 20(6):907-913. https://doi.org/10.1080/14787210.2022.2035217
Varikasuvu, SR, Thangappazham, B, Vykunta, A, Duggina, P, Manne, M, Raj, H, Aloori, S (2022a) COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther, 1–7
Velavan TP, Meyer CG (2020) Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 95:304–307
Velikova T, Fabbri A, Infante M (2021) The role of vitamin D as a potential adjuvant for COVID-19 vaccines. Eur Rev Med Pharmacol Sci 25:5323–5327
Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, Chugh S, Deshpande S, Ford C, Gama R (2013) Vitamin D: a negative acute phase reactant. J Clin Pathol 66:620–622
Watkins RR, Lemonovich TL, Salata RA (2015) An update on the association of vitamin D deficiency with common infectious diseases. Can J Physiol Pharmacol 93:363–368
Xie J, Ding C, Li J, Wang Y, Guo H, Lu Z, Wang J, Zheng C, Jin T, Gao Y, He H (2020) Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol 92:2004–2010
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M (2001) Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86:888–894
Zhang H, Du F, Cao X-J, Feng X-L, Zhang H-P, Wu Z-X, Wang B-F, Zhang H-J, Liu R, Yang J-J, Ning B, Chen K, Huang Z-P (2021) Clinical characteristics of coronavirus disease 2019 (COVID-19) in patients out of Wuhan from China: a case control study. BMC Infect Dis 21:207
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M. S. Z., M. E., A. M. A., M. M. E.: conceptualization. M. S. Z., A. A. E., O. A. K., M. M. A.: methodology. H. A. E., A. B. E., A. B. A., H. M. F.: data collection. H. A. E., A. B. E., A. B. A., H. M. F.: screening. A. Y. A., A. S. F., R. E., E. A. A., O. A. A., T. M. A.: data extraction. M. E., A. M. A., M. M. E., O. A. A., T. M. A., H. W. A.: risk of bias. M. S. Z., S. M. H., E. A. A.: analysis. M. E., A. M. A., M. M. E., O. A. A., T. M. A., S. M. H., H. W. A.: writing — original draft preparation. A. Y. A., A. S. F., R. E., A. A. E., O. A. K., M. M. A.: writing — review and editing. A. A. E., O. A. K., M. M. A.: supervision. All authors reviewed the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaazouee, M.S., Eleisawy, M., Abdalalaziz, A.M. et al. Hospital and laboratory outcomes of patients with COVID-19 who received vitamin D supplementation: a systematic review and meta-analysis of randomized controlled trials. Naunyn-Schmiedeberg's Arch Pharmacol 396, 607–620 (2023). https://doi.org/10.1007/s00210-022-02360-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02360-x