Abstract
The global COVID-19 pandemic is underway. In recent weeks, several countries throughout the globe, and particularly in Europe, have experienced an exponential increase in the number of individuals infected with COVID-19, probably induced by a new variant of SARS-CoV-2, called the “Omicron variant.” Mass vaccination against COVID-19 continues worldwide. Are authorized mRNA vaccines effective against the new Omicron variant? Recently, several pharmaceutical companies have developed oral antiviral pills against SARS-CoV-2, i.e., molnupiravir and paxlovid, that inhibit SARS-CoV-2 viral replication by acting on the RNA polymerase of SARS-CoV. In pre-registration clinical trials, molnupiravir and paxlovid have shown excellent clinical efficacy results, but what impact will these new oral antiviral agents have against pandemic COVID-19? In what specific clinical situations are they preferred over other antivirals such as remdesivir? In this brief review, we explore these important aspects.
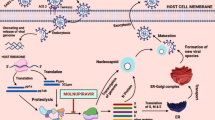
Graphical abstract

Similar content being viewed by others
Background
Pandemic COVID-19, current status, and Omicron variant
The COVID-19 global pandemic caused by SARS-CoV-2 is currently ongoing; to date recorded data indicate approximately 271 million individuals infected, and approximately 5.31 million deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reportsn.d.). SARS-CoV-2 is a member of the coronavirus family, viruses that are highly susceptible to mutation (Wang et al. 2020). Recently, a new variant of SARS-CoV-2 has been identified, named the “Omicron variant.” The Omicron variant was first identified on November 11, 2021, in Botswana, and on November 14, 2021, in South Africa. The World Health Organization on November 26, 2021, designated the B.1.1.529 variant (Omicron variant) as a variant of concern (VOC) (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concernn.d.). This may mean that the Omicron variant could likely be more infectious and dangerous than previous variants, and, potentially, having evidence of reduced expected outcomes. Omicron joins Delta, Alpha, Beta, Gamma, and Epsilon on the current WHO list of VOC. It is critical to identify whether the new variant has a greater ability to evade antibody neutralization developed by infections with previous variants or by vaccines. The Omicron variant has 37 amino acid mutations at the site of SARS-CoV-2 spike protein. Of the 37 mutations, 25 were found to be unique to this strain, while 12 were shared with the Alpha, Beta, Gamma, and Delta variants (Sarkar et al. 2021; Callaway and Ledford 2021; Pulliam et al. n.d.). The spike protein is the protein that mediates the attachment of SARS-CoV-2 to the host cell surface receptor angiotensin-converting enzyme-2 (ACE-2) allowing endocellular penetration of the virus and is the attack target of antibodies developed by mRNA vaccines and monoclonal antibodies (e.g., casirivimab-imdevimab) against SARS-CoV-2. Mutations in spike protein could therefore escape the efficacy of natural immunity or monoclonal antibodies. The apparent sharp increase in the incidence of Omicron variant cases in the South African province of Gauteng in November, and in central European countries during December, sounds like a wake-up call. Strong molecular evidence was urgently needed to clarify whether the Omicron variant spreads more easily from person to person than other variants, including Delta, and whether it is capable of generating more severe forms of COVID-19. Another important question is whether an individual cured of COVID-19 can become re-infected with the Omicron variant and to what extent available vaccines cover against the new variant. To date, there is still a paucity of sufficient data regarding the person-to-person transmissibility of the Omicron variant. However, there has been a rapid spread worldwide within days, and the number of cases has increased dramatically, showing an infection rate that is increasing faster than previous waves in any country. Some evidence has shown that infection rates were four times higher in the Omicron variant than in the wild type and twice as high in the Delta variant (Shanmugaraj et al. 2021; Dyer 2021). Genomic sequencing analysis of the Omicron variant showed that among the approximately 37 mutations affecting the spike protein (Callaway 2021; CDC 2021) and the N501Y mutation can increase the binding affinity to the ACE2 receptor, the endocellular gateway, which would be responsible for the increased transmission.
Drugs that can be used for the treatment of COVID-19 in the outpatient and home setting
Since the start of the pandemic, several drug treatments have been recommended for the outpatient setting and home management of SARS-CoV-2. Symptomatic medications such as acetaminophen or nonsteroidal anti-inflammatory drugs can be used in cases of fever or muscle pain. In general, the use of corticosteroids is recommended in hospitalized subjects with severe COVID-19 disease who require oxygen supplementation (Horby et al. 2020): outpatient use may be considered if the disease is progressing to the more severe form and if immediate hospitalization of the patient is not possible due to concomitant overload of hospital wards (WHO Living Guidance n.d.). To date, monoclonal antibodies and three antiviral treatments have been made available for the treatment of adults with COVID-19 with mild to moderate symptoms with concomitant risk factors who are not hospitalized and do not require additional oxygen therapy but who are at increased risk of progression to severe forms of COVID-19. The combination casirivimab/imdevimab and sotrovimab have been approved by the European Medicines Agency (EMA), and the combination bamlanivimab/etesevimab has been approved with emergency authorization status (EUA) (EMA 2022; FDA Emergency Use Authorization n.d.). Monoclonal antibodies are administered via a single intravenous infusion. It is noteworthy that the efficacy of monoclonal antibodies may be reduced against some viral variants: therefore, once the viral variant involved in the infection has been determined, it may be advantageous to have the opportunity to evaluate on which monoclonal antibody combination it is possible to direct the therapeutic choice (World Health Organization 2020). Currently, efficacy data on the Omicron variant indicate substantial ineffectiveness of bamlanivimab/etesevimab and casirivimab/imdevimab combinations, whereas sotrovimab seems to maintain adequate efficacy (Ferguson et al. 2021; Lewnard et al. 2022). Remdesivir, an antiviral drug administered by intravenous infusion, already approved by the EMA for the treatment of COVID-19 with pneumonia requiring supplemental oxygen therapy, was granted approval in December 2021 to extend its indication for the treatment of COVID-19 in the outpatient setting, in patients who do not require supplemental oxygen therapy but are at increased risk of progression to severe forms of COVID-19: the duration of treatment is 3 days, with a 200-mg loading dose on day 1 followed by a 100-mg dose on day 2 and day 3, respectively (EMA 2022; Ader et al. 2021; Gottlieb et al. 2021). Patients treated with monoclonal antibodies and remdesivir in an outpatient setting should be monitored, according to local practice, and drug administration should be performed under conditions in which severe hypersensitivity reactions, including anaphylaxis, can be treated (Chen et al. 2021). Recently, new oral antiviral agents such as molnupiravir and paxlovid that inhibit SARS-CoV-2 viral replication by acting on SARS-CoV-2 RNA polymerase have entered the licensed emergency phase (EMA 2022; FDA Emergency Use Authorization n.d.; Fan et al. 2021). These novel agents represent an additional weapon at our disposal in the battle against the COVID-19 pandemic and may ensure more compliant and safe patient intake in the home setting. Currently, especially in patients with concomitant diseases, clinical safety data of these new oral antivirals are still scarce: however, once new evidence becomes available, based on the new knowledge, it is desirable that the therapeutic choice is directed towards a specific treatment rather than another depending on the clinical condition of the individual patient (Wen et al. 2022a).
SARS-CoV-2 infection, new NVX-CoV2373 vaccine and antiviral oral agents
NVX-CoV2373 vaccine
On December 20, 2021, the EMA authorized a new NVX-CoV2373 vaccine with a different mode of action from the Pfizer and Moderna mRNA vaccines. The NVX-CoV2373 vaccine is a recombinant severe acute respiratory syndrome coronavirus 2 (rSARS-CoV-2) nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins. The NVXCoV2373 (Novavax) vaccine consists of 5 μg of a recombinant nanoparticle spike protein plus 50 μg of Matrix-M adjuvant. Early clinical data have demonstrated that a two-dose regimen administered at 3-week time intervals induces a robust immune response in healthy adult participants (Polack et al. 2020; Voysey et al. 2021). A phase 3, randomized, blinded, placebo-controlled study was performed to evaluate the efficacy, immunogenicity, and safety of NVX-CoV2373 in the prevention of COVID-19 in participants aged 18–84 years in the UK. A total of 15,187 participants were recruited. Data showed 86.3% efficacy against the B.1.1.7 (or alpha) variant and 96.4% efficacy against non-B.1.1.7 variants (Heath et al. 2021). Reactogenicity was generally mild, and the incidence of serious adverse events was similar in the Novavax group and placebo groups. Efficacy against the Omicron variant was not considered. Certainly, the new recombinant protein vaccine NVXCoV2373 (Novavax) represents a new weapon of prophylaxis against SARS-CoV-2.
Molnupiravir
The main therapeutic agents for the treatment of SARS-CoV-2 infection are immunomodulators/anti-inflammatory agents, anticoagulants, antivirals, and monoclonal antibodies. Recently, new oral antiviral agents such as molnupiravir and paxlovid have been approved and represent important therapeutic alternatives to remdesivir. Molnupiravir is an isopropyl derivative of the ribonucleoside analogue β-d-N4-hydroxycytidine (NHC); it is a prodrug that is immediately activated in its active form by plasma esterases. The active form of the drug then undergoes intracellular phosphorylation by host cell kinases to form an alternative substrate for viral RNA polymerase (Agostini et al. 2019). In vitro and in vivo tests show that molnupiravir was found to be a potent inhibitor of SARS-CoV-2 replication, (Fig. 1) (Zhao et al. 2021; Sheahan et al. 2021).
Molnupiravir mechanism of action against SARS-CoV-2. The EIDD-triphosphate form inhibits RNA replication of virus. Molnupiravir reduces the ability of SARS-CoV-2 to replicate in the body, producing alterations (mutations) of the genetic material (RNA) of the virus during replication, so as to make it unable to multiply
In addition, molnupiravir in phase I/II/III clinical trials demonstrated good efficacy and safety. Data indicate that molnupiravir reduced the risk of hospitalization or death by approximately 50% in non-hospitalized adults who had mild to moderate COVID-19 and were at risk for a serious disease outcome (Merck and Ridgeback’s 2021). In addition, molnupiravir has been shown to reduce the risk of hospitalization or death in all subgroups, with efficacy unaffected by the timing of symptom onset, underlying risk factors, or the type of SARS-CoV-2 variant (Gamma, Delta, and Mu). Another positive aspect is that the data demonstrate a generally good safety profile. The clinical trial showed that the incidence of any adverse event was comparable in the molnupiravir and placebo groups (35% and 40%, respectively), and the incidence of drug-related adverse events was also comparable (12% and 11%, respectively). In addition, fewer subjects discontinued study therapy because of an adverse event in the molnupiravir group compared with the placebo group (1.3% vs 3.4%) (Vitiello et al. 2021). Results from a clinical trial showed that 35.4% and 43.8% of individuals reported adverse events after administration of molnupiravir and placebo, respectively. No serious adverse events appeared, and the most commonly occurring non-serious adverse event was headache Molnupiravir (12.5%) and placebo (18.8%) (Painter et al. 2021). On March 23, 2022, molnupiravir received EUA from the Food and Drug Administration (FDA) (https://www.fda.gov/media/155053/downloadn.d.): the 800-mg daily dose for 5 days has been approved under EUA status as treatment for patients affected by COVID-19 and having mild-to-moderate symptoms. In another clinical trial, no serious adverse events were reported in all the patients who received 300- and 600-mg molnupiravir. The most common symptoms were loss of smell or taste, diarrhea, nausea, and cough (Khoo et al. 2021). The results of these trials revealed a well-tolerated safety profile of molnupiravir. To date, on ClinicalTrials.gov, there are 4 ongoing clinical trials evaluating the safety and efficacy of molnupiravir in patients with COVID-19 (Table 1). The results of the ongoing trial may further bring more clarity, especially on the efficacy of Molnupiravir in the new variant Omicron.
Paxlovid
The new oral antiviral agent paxlovid is a combination of two active ingredients. The first, nirmatrelvir (PF-07321332), acts by inhibiting SARS-CoV-2 viral replication by blocking the SARS-CoV-2-3CL protease; the second is ritonavir, an antiretroviral indicated for the treatment of HIV, which is also an inhibitor of cytochrome P4503A and CYP2D6 used to slow the metabolism of nirmatrelvir. The antiviral drug must be administered within 5 days of the onset of symptoms to stop the development of serious disease caused by the infection. In vitro studies confirm that nirmatrelvir is a potent 3CL protease inhibitor of the Omicron variant, suggesting that the drug demonstrates potential therapeutic efficacy against this novel variant as well (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additionalphase-23-study-resultsn.d.). On December 22, 2021, paxlovid received EUA from the FDA (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-receives-us-fdaemergency-use-authorizationn.d.). Pre-registration clinical trial results demonstrated an 89% reduction in the risk of hospitalization or mortality for COVID-19 compared with placebo. Among patients treated within the first 3 days of symptoms, at day 28 of follow-up, there were 3/389 hospitalizations with no deaths in the paxlovid group compared with 27/385 hospitalizations with 7 deaths in the placebo group (p < 0.0001) (https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oralantiviral-treatment-candidaten.d.). Several clinical trials are ongoing to evaluate the efficacy and safety of paxlovid in patients with COVID-19 (Table 1); an interesting study “Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR)” is evaluating the efficacy and safety of paxlovid administered twice daily for 5 days as a treatment of adults with acute COVID-19 (https://clinicaltrials.gov/ct2/show/study/NCT04960202?term=EPICHR&draw=2&rank=1n.d.). This is a placebo-controlled, double-blind study in patients who are at increased risk of developing severe COVID-19 disease. However, there is a risk of drug interactions in patients on polypharmacy due to the ritonavir component of paxlovid, a particularly potent inhibitor of CYP3A enzymes of the cytochrome P450 system (Hsu et al. 1998). Interaction between ritonavir and CYP3A-dependent drugs may result in increases in the area under the curve of blood concentrations of numerous drugs and possibly increased risk of adverse reactions (Hsu et al. 1998; https://www.fda.gov/media/155050/download2021). Many frail patients such as organ transplant recipients and those on immunosuppressant therapy and paxlovid are at risk for pharmacokinetic drug interactions because drugs such as cyclosporine, tacrolimus, and mTORi (sirolimus and everolimus) are highly dependent on CYP3A metabolism. Other drugs at risk for interactions with ritonavir include statins, calcium channel blockers, and anticoagulants such as warfarin (https://www.fda.gov/media/155050/download2021). Among the first countries in the world to have used large-scale application of the new oral antivirals is the state of Israel. Health authorities there reported that 92% of the 850 patients who took paxlovid showed marked improvement within 3 days; nearly 60% experienced relief of infection symptoms within a day; and, more importantly, none was hospitalized. In patients, paxlovid led to a rapid decrease in fever, headache, and cough. There were few but mild side effects caused by the drug (Fig. 2). To date, there are still few data in the literature on the safety of using the new oral antivirals against COVID-19; a recent meta-analysis considered a total of eight studies, showing that the new oral antivirals were effective in reducing mortality and hospitalization rates in patients with COVID-19. In addition, the antivirals did not increase the occurrence of adverse events, thus showing good overall safety. (Wen et al. 2022b).
Paxlovid’s mechanism of action includes 2 drugs, nirmatrelvir that is a peptidomimetic inhibitor of the 3CL protease: 3CL protease inhibition makes the protein incapable of processing polyprotic precursors and this determines the prevention of viral replication, and ritonavir, that inhibits the metabolism of Nirmatrelvir mediated by CYP3A, thus providing an increase plasma concentrations of Nirmatrelvir
Discussion
The COVID-19 pandemic has powerfully entered a new phase, primarily caused by the Omicron variant. Mass vaccination continues unabated worldwide. Early data show that mRNA vaccines also cover against infection with the Omicron variant; however, more data are needed in this direction. In the meantime, Pfizer has stated that the mRNA vaccine against the Omicron variant will be available in Europe in spring 2022 (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variantn.d.). In addition, on December 20, 2021, the EMA authorized a new NVX-CoV2373 vaccine with a different mode of action from the Pfizer and Moderna mRNA vaccines. In addition, new oral direct antiviral drugs against SARS-CoV-2 have recently been licensed, and the first available evidence is very positive. As of spring 2021, the most important question is whether and when SARS-CoV-2 will attenuate its contagiousness and become symbiotic with humans perhaps evolving into a common cold virus (Geoghegan and Holmes 2018). In this regard, the Omicron variant seems to suggest that this may happen soon. However, oral antiviral drugs and monoclonal antibodies must also be used at the right time and in the right doses, to avoid viral phenomena of drug resistance (Milestones 2020). Resistance to drugs, antivirals, antibiotics, or anticancer drugs, emerge due to the selection of variants that escape the action of drug treatment. Vaccines, on the other hand, prophylactically tend to induce host immune responses against multiple targets of the pathogen, whereas drugs target one or a few molecular targets (Kennedy and Read 2017). Consequently, viruses generate less variation for vaccine use than drug resistance (Vitiello 2021; Kennedy and Read 2018). The widespread of COVID-19 can be better monitored through diagnostic tests that allow a rapid molecular diagnosis The ability to adapt rapid diagnostic kits to detect new virus variants extends the diagnostic limitations (Domenico et al. 2021a; Domenico et al. 2021b). Coronaviruses are single-stranded RNA viruses and evolutionarily produce a significant proportion of mutations in the shortest amount of time compared with DNA viruses. The question is therefore whether new harmful variants will be blocked by vaccines and antivirals, and whether their use will favor the evolution of more benign strains, with selection in favor of lower virulence and especially lower lethality. However, it must be emphasized that if the current available mRNA vaccines lose their effectiveness (in this regard, some emerging data in Israel indicate limited effectiveness of booster with the fourth dose of mRNA vaccines), their technology allows them to be updated quickly. The world population hopes that, thanks to vaccines and newly available antivirals, the problem will be solved in a short time and that it is not just shifted and postponed until the next SARS-CoV-2 variant emerges.
Conclusions
Despite significant progress in understanding the pathophysiology of COVID-19 infection and new therapeutic strategies available, the number of new cases is increasing mainly due to the new Omicron variant. The contagiousness and lethality of this new variant are not fully known; investigations are ongoing to obtain further evidence. In the meantime, the recommendations considered so far in the scientific world, such as continuous vaccination, social distancing, and good use of available drugs, must continue to be followed. Recently, new antivirals directed against SARS-CoV-2 have been licensed, and new vaccines have been approved or are in the process of being approved, representing further powerful weapons available for the final victory against the COVID-19 pandemic. Oral direct antivirals have demonstrated excellent efficacy in pre-registration clinical trials; however, further well-designed randomized clinical trials with large sample sizes and longer follow-up periods are still needed in the future to confirm the efficacy and safety of molnupiravir and paxlovid in patients with COVID-19.
Data availability
Research data are not shared.
References
Ader F, Bouscambert-Duchamp M, Hites M et al (2021) Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(21)00485-0
Agostini ML, Pruijssers AJ, Chappell JD et al (2019) Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol 93(24):301348–301419. https://doi.org/10.1128/JVI.01348-19
Callaway E (2021) Heavily mutated Omicron variant puts scientists on alert. Nature News. https://www.nature.com/articles/d41586-021-03552-w. Accessed 16 Dec 2021
Callaway E, Ledford H (2021) How bad is Omicron? What Scientists Know so Far. Nature 600(7888):197–199. https://doi.org/10.1038/d41586-021-03614-z
CDC (2021) Science Brief: Omicron (B.1.1.529) Variant. CDC. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html. Accessed 16 Dec 2021
Chen P et al (2021) SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2029849
Di Domenico M, De Rosa A, Boccellino M (2021a) Detection of SARS-COV-2 Proteins Using an ELISA Test. Diagnostics (basel) 11(4):698
Di Domenico M, De Rosa A, Di Gaudio F et al (2021b) Diagnostic Accuracy of a New Antigen Test for SARS-CoV-2 Detection. Int J Environ Res Public Health 18(12):6310
Dyer O (2021) Covid-19: South Africa’s surge in cases deepens alarm over omicron variant. BMJ 375:n3013
EMA (2022) Treatments authorised in the European Union (EU) to treat COVID-19, following evaluation by the European Medicines Agency (EMA). https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-authorised. Accessed May 2022
Fan H, Lou F, Fan J, Li M, Tong Y (2021) The emergence of powerful oral anti-COVID-19 drugs in the post-vaccine era. Lancet Microbe. https://doi.org/10.1016/S2666-5247(21)00278-0
FDA Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs. 2022. Accessed May 2022.
Ferguson N, Ghani A, Hinsley W, Volz E (2021) team obotICC-r. Report 50: Hospitalisation risk for Omicron cases in England 2021. Available from: https://www.imperial.ac.uk/media/imperialcollege/medicine/mrc-gida/2021-12-22-COVID19-Report-50.pdf
Geoghegan JL, Holmes EC (2018) The phylogenomics of evolving virus virulence. Nat Rev Genet 19(12):756–769
Gottlieb RL, Vaca CE, Paredes R et al (2021) Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. https://doi.org/10.1056/nejmoa2116846
Heath PT, Galiza EP, Baxter DN et al (2021) Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med 385(13):1172–1183. https://doi.org/10.1056/NEJMoa2107659
RECOVERY Collaborative Group, Horby P, Lim WS et al (2020) Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. https://doi.org/10.1056/NEJMoa2021436
Hsu A, Granneman GR, Bertz RJ (1998) Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35(4):275–91. https://doi.org/10.2165/00003088199835040-00002
https://clinicaltrials.gov/ct2/show/study/NCT04960202t?term=EPIC-HR&draw=2&rank=1. Accessed 16 Dec 2021
https://www.fda.gov/media/155050/download. Accessed 12/24/2021
https://www.fda.gov/media/155053/download. 2022. Accessed Jan 2022
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant. 2022. Accessed May 2022.
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additionalphase-23-study-results. Accessed 21 Dec 2021
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-receives-us-fdaemergency-use-authorization novel#:~:text=The%20U.S.%20Food%20and%20Drug%20Administration%20(FDA)%20has%2 0issued%20an,at%20least%2040%20kg)%20with accessed 12/22/2021
https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oralantiviral-treatment-candidate. Accessed 21 Dec 20121
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. 2021. Accessed Dec 2021
https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. 2021. Accessed Dec 2021
Kennedy DA, Read AF (2017) Why does drug resistance readily evolve but vaccine resistance does not? Proc Royal Soc b 284:20162562
Kennedy DA, Read AF (2018) Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc Natl Acad Sci USA 115(51):12878–12886
Khoo SH, FitzGerald R, Fletcher T et al (2021) Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase 1, dose-escalating, randomised controlled study. medRxiv, 2021.2005.2003.21256309
Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY (2022) Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv. 2022:2022.01.11.22269045
Merck and Ridgeback’s (2021) Merck and Ridgeback’s investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate COVID-19 in positive interim analysis of phase 3 study. https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/. Accessed 20 Oct 2021
Milestones (2020) Vaccines, Nature Milestones
Painter WP, Holman W, Bush JA et al (2021) Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother 65(5):e02428-20. https://doi.org/10.1128/AAC.02428-20
Polack FP, Thomas SJ, Kitchin N et al (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(2603–15):5
Pulliam JRC et al (n.d.) Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021. https://doi.org/10.1101/2021.11.11.21266068
Sarkar R et al (2021) S glycoprotein diversity of the Omicron Variant. medRxiv preprint. https://doi.org/10.1101/2021.12.04.21267284
Shanmugaraj B, Malla A, Khorattanakulchai N, Phoolcharoen W (2021) SARS-CoV-2 omicron variant: could it be another threat? J Med Virol 94(4):1284–1288, 2022 04
Sheahan TP, Sims AC, Zhou S et al (2021) An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 12(541):eabb5883. https://doi.org/10.1126/scitranslmed.abb5883
Vitiello A (2021) Sars-Cov-2 and risk of antiviral drug resistance [published online ahead of print, 2021 Oct 29]. Ir J Med Sci. 1–2. https://doi.org/10.1007/s11845-021-02820-y
Vitiello A, Troiano V, La Porta R (2021) What will be the role of molnupiravir in the treatment of COVID-19 infection? [published online ahead of print, 2021 Nov 5]. Drugs Ther Perspect 1–2. https://doi.org/10.1007/s40267-021-00879-2
Voysey M, Clemens SAC, Madhi SA et al (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARSCoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99111
Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM (2020) SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol 10:587269. https://doi.org/10.3389/fcimb.2020.587269
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, Wang M, Mao Q (2022a) Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann Med 54(1):516–523. https://doi.org/10.1080/07853890.2022.2034936
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, Wang M, Mao Q (2022b) Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med 54(1):516–523. https://doi.org/10.1080/07853890.2022.2034936
WHO Living Guidance (n.d.) Corticosteroids for COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. 2022. Accessed May 2022
World Health Organization (2020) A living WHO guideline on drugs for covid-19. BMJ 370:m3379
Zhao J, Guo S, Yi D et al (2021) A cell-based assay to discover inhibitors of SARS-CoV-2 RNA dependent RNA polymerase. Antiviral Res 190:105078. https://doi.org/10.1016/j.antiviral.2021.105078
Author information
Authors and Affiliations
Contributions
AV: Conceptualization, writing—original draft.
RLP: Validation, reviewing and editing.
UT: Methodology, original draft preparation.
FF: Writing, review and editing; supervision.
AZ: Review and editing, visualization.
AMA: Validation, reviewing and editing.
MDD: Validation, reviewing and editing.
MB: Validation, Reviewing and Editing.
All authors read and approved the manuscript, and all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
The authors consent to the publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vitiello, A., La Porta, R., Trama, U. et al. Pandemic COVID-19, an update of current status and new therapeutic strategies. Naunyn-Schmiedeberg's Arch Pharmacol 395, 1159–1165 (2022). https://doi.org/10.1007/s00210-022-02265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02265-9