Abstract
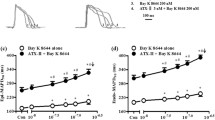
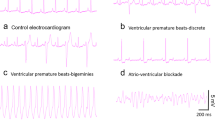
Aloperine (ALO), a quinolizidine alkaloid extracted from Sophora alopecuroides L., modulates hypertension, ventricular remodeling, and myocardial ischemia. However, few studies have evaluated the effects of ALO on other cardiovascular parameters. Accordingly, in this study, we used a rat model of aconitine-induced ventricular arrhythmia to assess the effects of ALO. Notably, ALO pretreatment delayed the onset of ventricular premature and ventricular tachycardia and reduced the incidence of fatal ventricular fibrillation. Moreover, whole-cell patch-clamp assays in rats’ ventricular myocyte showed that ALO (3, 10, and 30 μM) significantly reduced the peak sodium current density of voltage-gated Na+ channel currents (INa) in a concentration-dependent manner. The gating kinetics characteristics showed that the steady-state activation and recovery curve were shifted in positive direction along the voltage axis, respectively, and the steady-state inactivation curve was shifted in negative direction along the voltage axis, i.e., which was similar to the inhibitory effects of amiodarone. These results indicated that ALO had anti-arrhythmic effects, partly attributed to INa inhibition. ALO may act as a class I sodium channel anti-arrhythmia agent.








Similar content being viewed by others
Change history
20 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00210-021-02138-7
References
Marban E (2002) Cardiac channelopathies. Nature. 415:213–218
Xu J, Yang Y (2009) Traditional Chinese medicine in the Chinese health care system. Health Policy 90:133–139
Wang R, Deng X, Gao Q, Wu X, Han L, Gao X, Zhao S, Chen W, Zhou R, Li Z, Bai C (2020a) Sophora alopecuroides L.: an ethnopharmacological, phytochemical, and pharmacological review. J Ethnopharmacol 248:112172
Li Y, Wang G, Liu J, Ouyang L (2020b) Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: bioactivities, structure-activity relationships and preliminary molecular mechanisms. Eur J Med Chem 188:111972
Rong ZJ, Hu GS, Lin SY, Yan T, Li N, Zhao Y, Jia JM, Wang AH (2020) Constituents from the seeds of Sophora Alopecuroides L. Molecules. 25(2):411
Wang HQ, Xia CB, Chen L, Zhao JJ, Tao WW, Zhang X, Wang JH, Gao XJ, Yong JJ, Duan JA (2019) Phytochemical information and biological activities of quinolizidine alkaloids in sophora: a comprehensive review. Curr Drug Targets 20:1572–1586
Perez M, Espadinha M, Santos-Maria MM (2015) Indolo[2,3-a] quinolizidines and derivatives: bioactivity and asymmetric synthesis. Curr Pharm Des 21:5518–5546
Zhao XL, Gu DF, Qi ZP, Chen MH, Wei T, Li BX, Yang BF (2009) Comparative effects of sophocarpine and sophoridine on hERG K+ channel. Eur J Pharmacol 607:15–22
Ho MH, Li H, Shawn GJ, Dong XH, Chen CH, Xie H (2016) Two ssmall molecules block oral epithelial cell invasion by Porphyromons gingivalis. PLoS One 11(2):e0149618
Muhammad T, Sakhawat A, Khan AA, Huang H, Khan HR, Huang YH, Wang J (2020) Aloperine in combination with therapeutic adenoviral vector synergistically suppressed the growth of non-small cell lung cancer. J Cancer Res Clin Oncol 146:861–874
Wang H, Yang S, Zhou H, Sun M, Du L, Wei M, Luo M, Huang J, Deng H, Feng Y, Huang J, Zhou Y (2015) Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mechanisms. J Hematol Oncol 8:26
Lv XQ, Zou LL, Tan JL, Li H, Li JR, Liu NN, Dong B, Song DQ, Peng ZG (2020) Aloperine inhibits hepatitis C virus entry into cells by disturbing internalisation from endocytosis to the membrane fusion process. Eur J Pharmacol 883:173323
Ye Y, Wang Y, Yang Y, Tao L (2020) Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflamm Res 69(4):375–383
Zhao J, Zhang G, Li M, Luo QH, Yu L, Xu L (2018) Neuro-protective effects of aloperine in an Alzheimer’s disease cellular model. Biomed Pharmacother 108:137–143
Huang BW, Xiong JC, Zhao XY, Zheng YH, Zhu N (2020) Network pharmacology-based analysis of the pharmacological mechanisms of aloperine on cardiovascular disease. Evid Based Complement Alternat Med 2020:5180716
Mao Q, Guo FF, Liang XL, Wu YF, Lu YX (2019) Aloperine activates the PI3K/Akt pathway and protects against coronary microembolisation-induced myocardial injury in rats. Pharmacology. 104:90–97
Li WW, Li YS, Zhao Y, Ren LN (2020a) The protective effects of aloperine against ox-LDL-induced endothelial dysfunction and inflammation in HUVECs. Artif Cells Nanomed Biotechnol 48(1):107–115
Song SB, Chen YM, Han F (2018) Aloperine activates the Nrf2-ARE pathway when ameliorating early brain injury in a subarachnoid hemorrhage model. Exp Ther Med 15:3847–3855
Ren DL, Ma WS, Guo BZ (2017) Aloperine attenuates hydrogen peroxide-induced injury via anti-apoptotic activity and suppression of the nuclear factor-κB signaling pathway. Exp Ther Med 13:315–320
Chatterjee D, Pieroni M, Fatah M, Charpentier F, Cunningham KS, Spears DA, Chatterjee D, Suna G, Bos JM, Ackerman MJ, Schulze-Bahr E, Dittmann S, Notarstefano PG, Bolognese L, Duru F, Saguner AM, Hamilton RM (2020) An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. Eur Heart J 41(30):2878–2890
Manville RW, van der Horst J, Redford KE, Katz BB, Jepps TA, Abbott GW (2019) KCNQ5 activation is a unifying molecular mechanism shared by genetically and culturally diverse botanical hypotensive folk medicines. Proc Natl Acad Sci U S A 116(42):21236–21245
Kline CF, Mohler PJ (2009) From fifth business to protagonist: the complex roles of ion channel anchors in cardiac arrhythmia. Drug Discov Today Dis Model 6(3):63–69
Liu BX, Li S, Su Y, Xiong MT, Xu YW (2014) Comparative study of the protective effects of terfenadine and amiodarone on barium chloride/aconitine-induced ventricular arrhythmias in rats: a potential role of terfenadine. Mol Med Rep 10(6):3217–3226
Guinamard R, Hof T, Sallé L (2014) Current recordings at the single channel level in adult mammalian isolated cardiomyocytes. Methods Mol Biol 1183:291–307
Coulson JM, Caparrotta TM, Thompson JP (2017) The management of ventricular dysrhythmia in aconite poisoning. Clin Toxicol (Phila) 55(5):313–321
Shaw RM, Rudy Y (1997) Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81:727–741
Molinarolo S, Lee S, Leisle L (2018) Cross-kingdom auxiliary subunit modulation of a voltage-gated sodium channel. J Biol Chem 293:4981–4992
Goldin AL (2002) Evolution of voltage-gated Na (+) channels. J Exp Biol 205:575–584
Wu F, Yao WX, Yang JM (2017) Protective effects of aloperin on monocroline-induced pulmonary hypertension via regulation of Rho A/Rho kinsase pathway in rats. Biomed Pharmacother 95:1161–1168
Nergui S, Fukumoto Y, Do EZ, Nakajima S, Shimizu T, Ikeda S, Elias-Al-Mamun M, Shimokawa H (2014) Role of endothelial nitric oxide synthase and collagen metabolism in right ventricular remodeling due to pulmonary hypertension. Circ J 78(6):1465–1474
Lu HR, Yang P, Remeysen P (1999) Ischemia/reperfusion-induced arrhythmias in anaesthetized rats: a role of Na+ and Ca2+ influx. Eur J Pharmacol 365:233–239
Valdivia CR, Chu WW, Pu J (2005) Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38:475–483
Zhou S, Li S, Liu Y, Liu X (2008) Sodium channel and its role in drug research progress. J Med Rep 7:822–823
Fu M, Wu M, Wang JF (2007) Disruption of the entracelular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes. Biochem Biophys Res Commun 354(4):929–936
Wang XC, Jia QZ, Yu YL (2020b) Inhibition of the I and the activation of peak I contribute to the arrhythmogenic effects of aconitine and mesaconitine in guinea pigs. Acta Pharmacol Sin 0:1–2
Lu HR, De CF (1993) R 56 865, a Na+/Ca(2+)-overload inhibitor, protects against aconitine-induced cardiac arrhythmias in vivo. J Cardiovasc Pharmacol 22(1):120–125
Funding
This work was supported by the Research and Innovation Program for College Students of Yangzhou University (grant number X20200782).
Author information
Authors and Affiliations
Contributions
M.T.L. designed the study. Z.X.X. conducted the experiments. M.T.L. and Y.Y.D. analyzed data and performed the animal research. M.T.L., F.Z., Y.R.W., Y.W.G., X.T.H., and Y.Z.D. performed the cell research. M.T.L. wrote the manuscript. All authors have read and approved the manuscript. All authors confirm that all data were generated in-house, and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The Ethics Committee of Yangzhou University Medical College approved the experimental protocols (Yangzhou, China).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Aloperine pretreatment delayed the onset of VP and VT.
• Aloperine also decreased the incidence of VF.
• Aloperine blocked voltage-gated Na+ channels in a concentration-dependent manner.
• Aloperine may have uses as a class I sodium channel anti-arrhythmia agent.
Rights and permissions
About this article
Cite this article
Li, Mt., Du, Yy., Zhong, F. et al. Inhibitory effects of aloperine on voltage-gated Na+ channels in rat ventricular myocytes. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1579–1588 (2021). https://doi.org/10.1007/s00210-021-02076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02076-4