Abstract
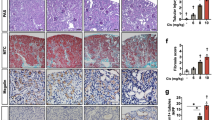
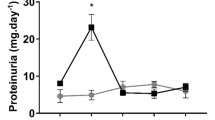
Cisplatin (CP) is nephrotoxic, and this side effect is used as an animal model for acute kidney injury (AKI). Earlier research has been focused on CP-induced AKI, with relatively little attention being paid to its ability to progress to chronic kidney disease (CKD) on repeated administration. We aimed here to test the dose dependency of its nephrotoxic actions by comparing various physiological, biochemical, molecular, and histopathological indices using repeated increasing doses of CP in rats. Furthermore, we investigated whether these doses of CP would result in the development of CKD. Biochemical, molecular, and histopathological measurements were conducted in plasma, urine, and/or kidneys of rats treated with increasing doses of CP at 1.6, 3.2, and 4.8 mg kg−1 weekly for four consecutive weeks. These doses induced significant and dose-dependent elevations in most of the measured renal indices. These included increased renal fibrosis, as suggested histopathologically and biochemically by the significant increase in transforming growth factor-β1, significant decrease in actin alpha 2, and variable actions of collagen I and IV. CP also dose-dependently increased nuclear factor (erythroid-derived 2)-like 2 and caspase-3. Multiple repeated doses of CP (1.6 to 4.8 mg kg−1) induced multiple episodes of AKI, leading to CKD after the 4th weekly dose and confirmed that this dosage regimen could be used as an experimental animal model of AKI progressing to CKD. These actions were driven by inflammation, oxidative, and nitrosative stress.






Similar content being viewed by others
References
Abdelrahman AM, Al Suleimani Y, Shalaby A, Ashique M, Manoj P, Nemmar A, Ali BH (2019) Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice. Naunyn Schmiedeberg's Arch Pharmacol 392:45–53
Alexakis C, Maxwell P, Bou-Gharios G (2006) Organ-specific collagen expression: implications for renal disease. Nephron Exp Nephrol 102:e71–e75
Ali BH, Ahmed IH (2006) Hormonal replacement therapy in an animal model with chronic renal failure and gonadectomy: biochemical and hematological study. Ren Fail 28:331–335
Ali BH, Abdelrahman AM, Al-Salam S, Sudhadevi M, AlMahruqi AS, Al-Husseni IS, Beegam S, Dhanasekaran S, Nemmar A, Al-Moundhri M (2011) The effect of sildenafil on cisplatin nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 109:300–308
Ali BH, Al Za’abi M, Shalaby A, Manoj P, Waly MI, Yasin J, Fahim M, Nemmar A (2015) The effect of thymoquinone treatment on the combined renal and pulmonary toxicity of cisplatin and diesel exhaust particles. Exp Biol Med (Maywood) 240:1698–1707
Ali BH, Karaca T, Al Suleimani Y, Al Za’abi M, Al Kalbani J, Ashique M, Nemmar A (2017) The effect of swimming exercise on adenine-induced kidney disease in rats, and the influence of curcumin or lisinopril thereon. PLoS One 12:e0176316
Ali BH, Al Salam S, Al Suleimani Y, Al Za’abi M, Abdelrahman AM, Ashique M, Manoj P, Adham SA, Hartmann C, Schupp N, Nemmar A (2019) Effects of the SGLT-2 inhibitor canagliflozin on adenine-induced chronic kidney disease in rats. Cell Physiol Biochem 52:27–39
Al Za’abi M, Ali B, Al Toubi M (2013) HPLC-fluorescence method for measurement of the uremic toxin indoxyl sulfate in plasma. J Chromatogr Sci 51:40–43
Al Za’abi M, Al Salam S, Al Suleimani Y, Manoj P, Nemmar A, Ali BH (2018) Gum acacia improves renal function and ameliorates systemic inflammation, oxidative and nitrosative stress in streptozotocin-induced diabetes in rats with adenine-induced chronic kidney disease. Cell Physiol Biochem 45:2293–2304
Anand S, Caplin B, Gonzalez-Quiroz M, Schensul SL, Bhalla V, Parada X, Nanayakkara N, Fire A, Levin A, Friedman DJ, International Society of Nephrology’s International Consortium of Collaborators on Chronic Kidney Disease of Unknown Etiology (i3C) (2019) Epidemiology, molecular, and genetic methodologies to evaluate causes of CKD around the world: report of the working group from the ISN international consortium of collaborators on CKD. Kidney Int 96:1254–1260
Armutcu F, Demircan K, Yildirim U, Namuslu M, Yagmurca M, Celik HT (2019) Hypoxia causes important changes of extracellular matrix biomarkers and ADAMTS proteinases in the adriamycin-induced renal fibrosis model. Nephrology (Carlton) 24:863–875
Badid C, Desmouliere A, Babici D, Hadj-Aissa A, McGregor B, Lefrancois N, Touraine JL, Laville M (2002) Interstitial expression of alpha-SMA: an early marker of chronic renal allograft dysfunction. Nephrol Dial Transplant 17:1993–1998
Campaner AB, Ferreira LM, Gragnani A, Bruder JM, Cusick JL, Morgan JR (2006) Upregulation of TGF-beta1 expression may be necessary but is not sufficient for excessive scarring. J Invest Dermatol 126:1168–1176
Eddy AA (2014) Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl 4:2–8
He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, Sun L, Peng Y, Liu F, Venkatachalam MA, Dong Z (2017) AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int 92:1071–1083
Hoek J, Bloemendal KM, van der Velden LA, van Diessen JN, van Werkhoven E, Klop WM, Tesselaar ME (2016) Nephrotoxicity as a dose-limiting factor in a high-dose CP-based chemoradiotherapy regimen for head and neck carcinomas. Cancers (Basel) 8: E21
Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL (2019) Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci 20:E3011
James MT, Pannu N, Hemmelgarn BR, Austin PC, Tan Z, McArthur E, Manns BJ, Tonelli M, Wald R, Quinn RR, Ravani P, Garg AX (2017) Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA 318:1787–1797
Karasawa T, Steyger PS (2015) An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 237:219–222
Ko YJ, Sugar L, Zaltzman J, Paul LC (1997) Alpha-smooth muscle actin and collagen deposition in dysfunctional renal transplants. Transplantation 63:156–158
Koyner JL, Zarbock A, Basu RK, Ronco C (2019) The impact of biomarkers of acute kidney injury on individual patient care. Nephrol Dial Transplant 14:gfz188
Iwata K, Watanabe H, Morisaki T, Matsuzaki T, Ohmura T, Hamada A, Saito H (2007) Involvement of indoxyl sulfate in renal and central nervous system toxicities during cisplatin-induced acute renal failure. Pharm Res 24:662–671
Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD (2016) Long-term renal outcomes after CP treatment. Clin J Am Soc Nephrol 11:1173–1179
Li L, Zepeda-Orozco D, Black R, Lin F (2010) Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol 176:1767–1778
Li XW, Feng LX, Zhu XJ, Liu Q, Wang HS, Wu X, Yan P, Duan XJ, Xiao YQ, Cheng W, Peng JC, Zhao F, Deng YH, Duan SB (2020) Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed Pharmacother 121:109662
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev 7:684–696
Liu JQ, Cai GY, Wang SY, Song YH, Xia YY, Liang S, Wang WL, Nie SS, Feng Z, Chen XM (2018) The characteristics and risk factors for cisplatin-induced acute kidney injury in the elderly. Ther Clin Risk Manag 14:1279–1285
Loeffler I, Wolf G (2014) Transforming growth factor-β and the progression of renal disease. Nephrol Dial Transplant 29(Suppl 1):i37–i45
Meng XM, Nikolic-Paterson DJ, Lan HY (2014) Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10:493–503
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
National Kidney Foundation (2019) 37 million American adults now estimated to have chronic kidney disease. https://www.kidney.org/news/37-million-american-adults-now-estimated-to-have-chronic-kidney-disease. Accessed 24 July 2020
Nguyen MT, Devarajan P (2008) Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol 23:2151–2157
Ojima T, Nakamura M, Nakamori M, Katsuda M, Hayata K, Kitadani J, Maruoka S, Shimokawa T, Yamaue H (2019) Triplet chemotherapy with docetaxel, cisplatin and S-1 for unresectable advanced squamous cell carcinoma of the esophagus: phase I/II trial results. Oncotarget 10:847–855
Parrish AR (2016) The cytoskeleton as a novel target for treatment of renal fibrosis. Pharmacol Ther 166:1–8
Perše M, Večerić-Haler Ž (2018) Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed Res Int 12:1462802
Rasmussen DGK, Boesby L, Nielsen SH, Tepel M, Birot S, Karsdal MA, Kamper AL, Genovese F (2019) Collagen turnover profiles in chronic kidney disease. Sci Rep 9:16062
Sharp CN, Siskind LJ (2017) Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol 313:F835–F841
Sharp CN, Doll MA, Dupre TV, Shah PP, Subathra M, Siow D, Arteel GE, Megyesi J, Beverly LJ, Siskind LJ (2016) Repeated administration of low-dose cisplatin in mice induces fibrosis. Am J Physiol Renal Physiol 310:F560–F568
Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ (2018) Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Renal Physiol 315:F161–F172
Skrypnyk NI, Siskind LJ, Faubel S, de Caestecker MP (2016) Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol 310:F972–F984
Strutz F, Zeisberg M (2006) Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol J Am Soc Nephrol 17:2992–2998
Szturz P, Cristina V, Herrera Gómez RG, Bourhis J, Simon C, Vermorken JB (2019) Low-dose vs. high-dose cisplatin: lessons learned from 59 chemoradiotherapy trials in head and neck cancer. Front Oncol 9:86
Uber AM, Sutherland SM (2019) Nephrotoxins and nephrotoxic acute kidney injury. Pediatr Nephrol (In press)
Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G (2014) The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 25:1897–1907
Wang CH, Lai YH, Kuo CH, Lin YL, Tsai JP, Hsu BG (2019) Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins (Basel) 11 pii: E589
Xu Y, Xie Y, Shao X, Ni Z, Mou S (2015) L-FABP: a novel biomarker of kidney disease. Clin Chim Acta 445:85–90
Acknowledgments
This work was supported by funds from the Sultan Qaboos University. Thanks are due to Professor Gerald Blunden for reading the MS and to the staff of the SQU Small Animal House for looking after the animals.
Author information
Authors and Affiliations
Contributions
BHA and MAZ conceived and designed the project. MA and PM conducted the experiments. SAS contributed to histopathology work. YAS and AN contributed to interpretation of data. BHA and MAZ wrote the manuscript. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
This research was approved by the Animal Research Ethics Committee of the Sultan Qaboos University (SQU/AEC/2019–20/11). Procedures involving animals and their care were carried out in conformity with international laws and policies (EEC Council directives 86/609, OJL 358, 12 December 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publications No. 85–23, 1985).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Za’abi, M., Al Salam, S., Al Suleimani, Y. et al. Effects of repeated increasing doses of cisplatin as models of acute kidney injury and chronic kidney disease in rats. Naunyn-Schmiedeberg's Arch Pharmacol 394, 249–259 (2021). https://doi.org/10.1007/s00210-020-01976-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01976-1