Abstract
Infections are a major problem in patients with burn diseases. Mortality is high despite antibiotic therapy as studies are controversial concerning drug underdosing. The aims of this prospective, observational study were to monitor plasma concentrations of piperacillin during standard piperacillin/tazobactam treatment in 20 burn patients and 16 controls from the intensive care unit (ICU) and to optimize doses by in silico analyses. Piperacillin/tazobactam (4/0.5 g, tid) was administered over 0.5 h. Blood samples were taken at 1, 4, and 7.5 h after the end of the infusion. Free piperacillin plasma concentrations were determined. Pharmacokinetic parameters and in silico analysis results were calculated using the freeware TDMx. The primary target was defined as percentage of the day (fT>1xMIC; fT>4xMIC) when piperacillin concentrations exceeded 1xMIC/4xMIC (minimum inhibitory concentration), considering a MIC breakpoint of 16 mg/L for Pseudomonas aeruginosa. In an off-label approach, two burn patients were treated with 8/1 g piperacillin/tazobactam, 3 h qid. fT>1xMIC (55 ± 22% vs. 77 ± 24%) and fT>4xMIC (17 ± 11% vs. 30 ± 11%) were lower in burn than in ICU patients after 4/0.5 g, 0.5 h, tid. In silico analyses indicated that fT>1xMIC (93 ± 12% burn, 97 ± 4% ICU) and fT>4xMIC (62 ± 23% burn, 84 ± 19% ICU) values increase by raising the piperacillin dosage to 8/1 g qid and prolonging the infusion time to 3 h. Off-label treatment results were similar to in silico data for burn patients (84%fT>1xMIC and 47%fT>4xMIC). Standard dosage regimens for piperacillin/tazobactam resulted in subtherapeutic piperacillin concentrations in burn and ICU patients. Dose adjustments via in silico analyses can help to optimize antibiotic therapy and to predict respective concentrations in vivo. Trial registration: NCT03335137, registered 07.11.2017, retrospectively.
Similar content being viewed by others
Introduction
Infections represent a major problem in burn patients and are associated with adverse outcomes and mortality (Chim et al. 2007; Rafla and Tredget 2011). The use of antibiotics for severe burns remains controversial, however, and standard guidelines do not recommend this treatment (Yoshino et al. 2016). While Avni et al. claimed that prophylaxis with systemic antibiotics significantly reduced all-cause mortality by 50%, Barajas-Nava et al. found no difference in all-cause mortality (Avni et al. 2010; Barajas-Nava et al. 2013). Very recently, Tagami et al. demonstrated that prophylactic antibiotics may improve survival in mechanically ventilated patients with severe burns but not in those who do not receive mechanical ventilation (Tagami et al. 2016). Conflicting results have been attributed to the methodological quality of the data but the question of whether sufficient antibiotic concentrations were reached by standard dosing regimens, thereby influencing clinical outcome, has not been adequately addressed. Pseudomonas aeruginosa (P. aeruginosa) infections are common in burn patients and impede the recovery process (Church et al. 2006). Indeed, this organism is responsible for bacteremia in wound cultures of burn patients (Mir et al. 2017) and is also associated with high mortality in both burn patients and patients on the intensive care unit (ICU; Mann et al. 2012; McManus et al. 1985; Shorr 2009; Tredget et al. 2004). Considering the presence of these dominant bacteria, piperacillin/tazobactam (PIP/TAZ) is commonly used in burn patients to treat infections, showing particularly good activity against Gram-negative pathogens, including P. aeruginosa. For PIP, as a time-dependent antibiotic, the duration (fT>1xMIC) for which the unbound drug concentration exceeds at least the minimum inhibitory concentration (MIC) is crucial for its antibacterial effects (Craig 1998). However, clinical evaluations have claimed that ß-lactam concentrations up to four times over the MIC are clinically more effective (Li et al. 2007; McKinnon et al. 2008).
Dose recommendations for PIP/TAZ treatment of burn patients are mainly based on pharmacokinetic (PK) data originating from healthy volunteers or noncritically ill patients (Roberts 2011). However, as PK parameters such as volume of distribution and clearance (Vd and CL) may be dramatically altered in burn patients, such a dosing regimen may produce only subtherapeutic concentrations of these antibiotics, thereby contributing to a lower survival (Weinbren 1999). PK parameters may be affected by the timing of events related to burn injuries: the first 48 h after injury are characterized by burn shock, capillary leak, severe hypovolemia, edema, hypoalbuminemia, and a low glomerular filtration rate (GFR) (Bourget et al. 1996). In contrast, during the hypermetabolic phase (> 48 h), the blood flow to tissues and organs (kidney and liver) is increased, thus enhancing GFR and clearance (CL) of PIP (Bourget et al. 1996). Hence, it seems reasonable that plasma concentrations are altered in burn patients as (1) changes in renal function may affect the PK of PIP, which is mainly eliminated via the kidney (Bergan 1981); (2) nonplasma-bound PIP may be altered due to hypoalbuminemia and the therapeutic need to supplement albumin (Cartotto and Callum 2012), protein binding for PIP in healthy volunteers is ~ 30% (Bergan 1981), and burn injury, in particular, is known to result in decreased albumin concentrations and altered plasma protein binding of drugs (Blanchet et al. 2008); and (3) initial fluid resuscitation is mandatory for patients with a total burn surface area (TBSA) > 20% as major burn causes tissue destruction with capillary leakage leading to plasma extravasation and edema. Edema formation is already seen in the first hour after burn injury, reaching peak values after 12–24 h. Thereafter, volume is reabsorbed and eliminated (Demling 2005).
Considering these PK changes, a modified dosing strategy may be important to optimize antibiotic efficacy. Various studies recommend therapeutic drug monitoring (TDM) to optimize the dosage of ß-lactams for critically ill patients (Dellinger et al. 2004; Patel et al. 2012; Roberts et al. 2010). The aim of our study was to determine whether standard treatment of burn patients with PIP/TAZ (4/0.5 g, infusion duration 0.5 h, tid) and patients on the ICU of the internal medicine department, serving as controls, can produce therapeutic concentrations of free (unbound) PIP in plasma. Secondly, we performed in silico dosage simulations to assess adjustments of the dosing regimen for burn patients to enhance PIP concentrations and improve antibiotic efficacy. Finally, we tested whether adjusting the PIP dose produces adequate plasma concentrations as predicted by in silico analysis, treating two burn patients with 8/1 g PIP/TAZ, qid in an off-label approach and prolonged infusion duration (3 h).
Materials and methods
Study design
This prospective, observational study (NCT03335137) was performed from May 2014 to May 2016 and was approved by the ethics committee of the University (reference number 13-275). Informed consent was obtained for all patients. Burn patients from the Burn Care Unit of Plastic Surgery were included in the study. Patients from the ICU suffering from pneumonic sepsis served as controls. The inclusion criteria for all patients for treatment with PIP/TAZ consisted of the clinical need for antibiotics for at least 3 days and, additionally for burn patients, a TBSA > 20% or presence of an inhalation trauma. Burn patients received pain medication as well as airway, fluid, and wound management (Alharbi et al. 2012). Burn patients received 4 g PIP as short-term (0.5 h) infusions every 8 h (= 4 g/0.5 h/tid) in a prophylactic approach before a microbiological diagnosis was made. Patients were co-treated with 0.5 g TAZ per 4 g PIP and the amount of TAZ was proportionally scaled up when PIP dosage was increased. To keep readability and comprehension as simple as possible, we did not include TAZ dosage in the remainder of the paper. Although dose reduction is recommended in cases of renal dysfunction, PIP/TAZ dosing was not adjusted since plasma analysis was not performed close to the time of treatment. Lactated Ringer’s solution (24.1 ± 3.1 L, ranging from 8.5 to 48.8 L) was infused in burn patients by giving half of the fluid in the first 8 h and the next half over the next 16 h, according to standard guidelines (Guilabert et al. 2016). This volume displacement within 3 days is supposed to affect PIP concentration, thus prompting us to estimate PIP plasma concentrations at d1 and d3. Blood samples (5 mL) were drawn into lithium heparin tubes 1, 4, and 7.5 h after starting the infusion during the 3rd dosing interval at d1 and the 7th dose interval at d3 (Fig. S1). Thus, six samples and a total volume of 30 mL were gained from each patient. Blood samples were centrifuged to separate plasma, which was stored at − 80 °C until analysis. ICU patients also received 4 g/0.5 h/tid PIP and blood samples were similarly achieved.
Two burn patients received PIP in an off-label approach. At d1, an initial loading dose of 4 g/0.5 h PIP was administered during initial burn blister debridement immediately after patients arrived at the burn intensive care station. Thereafter, they received 8 g/3 h/qid. At d2, both patients were treated with 4 g/3 h/qid. Blood samples were taken during each dose interval after 3 and 5.5 h at d1 and d2.
Analytical methods
Total PIP concentrations were determined in plasma using high-performance liquid chromatography (HPLC) and UV detection (λ = 254 nm), as previously described (McWhinney et al. 2010). To quantify the total PIP concentration, 200 μL plasma was buffered with 200 μL phosphate buffer (50 mM NaH2PO4, pH 6.0) and acetonitrile (400 μL) was added for protein precipitation, followed by a wash step with dichloromethane (2 mL). After centrifugation (3000g, 20 min), 10 μL of the aqueous layer were injected into the HPLC system, consisting of a Waters XBridge (C18 BEH 2.5 μm, 50 × 3 mm) column, a precolumn (Nucleoshell RP18 2.7 μm, 4 × 3mm Macherey-Nagel GmbH & Co. KG), and an acetonitrile phosphate-buffered mobile phase (500:125 mL, pH 6, 0.4 mL min−1, 40 °C) (McWhinney et al. 2010). The linearity for total PIP has been shown on spiked plasma between 2 and 200 mg/L. Unbound PIP concentrations were measured using ultrafiltration and HPLC, as described in the literature (Briscoe et al. 2012). Briefly, 300 μL plasma was buffered with 10 μL phosphate buffer (3 M KH2PO4, pH 7.5) and ultrafiltered using Nanosep Omega 10K filters (VWR, Ismaning, Germany) (Briscoe et al. 2012; Schleibinger et al. 2015). Then, 2 μL of the ultrafiltrate was injected into the HPLC system. The recovery rate after sample preparation was 103%. The linearity of this method was approved (ranging between 2 and 200 mg/L; r = 0.9992) and the detection limit was 2 mg/L. The bias of this method was < 5%.
Pharmacokinetic analysis
Considering parameters in burn and ICU patients [sex, age, body weight, height, serum creatinine (SCr), measured PIP concentration, protein binding, and MIC], we calculated Vd, CL, and half-life (t1/2) by using TDMx (TDMx—model-supported Therapeutic Drug Monitoring for Precision Dosing; Wicha et al. 2015). The eGFR was estimated by Cockroft-Gault as we did not sample 24-h urine to measure 24-h urinary creatinine clearance. To further assess an optimized PIP regimen for burn patients, we performed in silico analysis using TDMx by varying PIP doses, dose intervals, and infusion duration and by intraindividually considering the parameters sex, age, body weight, height, SCr, measured PIP concentration, protein binding, and MIC.
Pharmacokinetic-pharmacodynamic targeting
We used the EUCAST breakpoint (16 mg/L) for P. aeruginosa (The European Committee on Antimicrobial Susceptibility Testing - EUCAST 2017). According to others (Roberts et al. 2014), we calculated the time (% of day) during which free drug concentrations exceeded 1xMIC (fT>1xMIC) or 4xMIC (fT>4xMIC) by using the TDMx freeware. TDMx uses a population PK model in conjunction with a Bayesian engine to derive the individual pharmacokinetic parameters (McKinnon et al. 2008; Tam et al. 2002). We additionally counted the number of patients with values exceeding 50%fT>1xMIC (indicating the conservative PK/PD target), or 100%fT>1xMIC and 100%fT>4xMIC (more indicating an aggressive PK/PD target).
Statistical Analysis
Data are presented as scatterplots and in line graphs as means ± SD by Prism 6.0 (GraphPad Software, La Jolla, USA). The t test was used to test for significance between the two groups. A two-way ANOVA was performed considering the factor time and patient group. p values in the figures originated from two-way ANOVA by testing differences between burn and ICU patients or of dosing regimens. Paired two-way ANOVA was calculated for testing time differences between d1 and d3. Bonferroni’s post hoc test was only performed if F reached p < 0.05 and there was no significant variance inhomogeneity. Differences were considered to be significant at p < 0.05. Correlation analyses were performed by two-tailed Pearson test.
Results
Main study
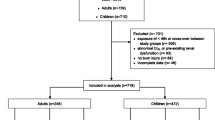
A total of 20 burn and 16 ICU control patients were included. Two burn patients and one ICU patient were excluded due to missing samples. One further burn patient was excluded from most evaluations due to a pre-existing, dialysis-dependent renal failure. Data of this patient were only included in evaluations showing a relationship between renal function and PK/PD parameters (Fig. 3). Demographic and clinical characteristics of burn and ICU patients are depicted in Table 1, showing that burn patients were obviously younger than ICU patients and that the burn group consisted of more males than females while the gender ratio was equal in the ICU patients. The median ABSI score was 8 and 3rd degree burn 33%. TISS scoring indicated that intensive care treatment requirements were higher for burn patients than for ICU patients (Table 1). CRP increased beyond normal concentrations in most patients (Table 1). Indeed, 21% of burn patients died during hospitalization. All of them had suffered an inhalation injury. Mortality showed a weak correlation with TBSA (r = 0.3041; p < 0.05). Most infections were wound infections with various different organisms, also including P. aeruginosa (Table 2). Furthermore, 44% of burn patients had sepsis according to the international consensus definitions for sepsis (Singer et al. 2016). Burn patients also received albumin (20%, in total 639 ± 72 mL, ranging from 100 to 1200 mL) at d2 and d3 after burn injury. The plasma albumin concentrations in burn patients were below the normal range (Jeon et al. 2014) (33–55 g/dL) at d1 and d3 (Table 1). Values at d3 were slightly higher than at d1 due to albumin supplementation.
In contrast to ICU patients, almost none of the burn patients achieved peak levels markedly exceeding the therapeutic target of 4xMIC breakpoint (Fig. 1a, b). In consequence, fT>4xMIC of burn patients was extremely low and was doubled in ICU patients, not differing between d1 and d3 (Fig. 1c, d). When absolute numbers of burn and ICU patients who reached 50%fT>MIC, 100%fT>MIC, or 100%fT>4xMIC after 4 g/0.5 h/tid PIP were counted, it also became evident that the efficacy of more aggressive antibiotic treatment is low, particularly in burn patients (Tab. S2). Plasma protein binding (21 vs. 13%) was slightly higher in the ICU patients as the free PIP was lower without revealing any differences between d1 and d3 (Fig. 2a). Linear protein binding was detected, as the linear regression line did not differ from zero (Fig. 2b, c). eGFR was higher in burn than in ICU patients, not differing in SCr (Table 3). This finding may also be related to the younger age of this group (Table 1) as age is a factor of the Cockcroft-Gault formula. Five burn patients had abnormal renal function (eGFR < 60 mL/min) and four of them died (Fig. 3d). Five showed an augmented renal clearance (ARC), defined as eGFR ≥ 130 mL/min. In contrast, only one ICU patient had ARC while renal function of the other ICU patients was normal or impaired. As eGFR was higher in burn patients, half-life was clearly lower and CL higher than in ICU patients (Table 3). Vd was ~ 30% higher in burn patients (Table 3). eGFR and fT>1xMIC and fT>4xMIC (Fig. 3a, b) as well as eGFR and CL correlated moderately (Fig. 3c) in burn but not in ICU patients. Thus, burn patients with renal dysfunction (< 60 mL/min) had a significantly higher probability of showing adequate drug concentrations than patients with a normal eGRF, while burn patients with ARC did not even achieve the target of fT>4xMIC (Fig. 3c).
Pharmacokinetic profiles of piperacillin (free concentrations) in burn and ICU patients at day 1 (a) and day 3 (b) after administration of 4 g/0.5/tid PIP. The dashed lines indicate the 1xMIC (16 mg/L) or 4xMIC (64 mg/L) of piperacillin for P. aeruginosa according to EUCAST (The European Committee on Antimicrobial Susceptibility Testing - EUCAST 2017). Subfigures (c) and (d) depict the time (% of 24 h, calculated by using the web-based TDMx-Software (TDMx - model-supported Therapeutic Drug Monitoring for Precision Dosing; Wicha et al. 2015)) when the free drug concentration exceeds the 1xMIC (fT>1xMIC) and 4xMIC (fT>4xMIC) for P. aeruginosa in burn and ICU patients. A 2-way ANOVA was performed to examine the effects of patients (burn vs. ICU) and time (d1 vs. d3). Values in line graphs are depicted as means ± SD; *p < 0.05 vs. ICU
Plasma protein binding of piperacillin. A two-ANOVA was performed to examine the effects of patients (burn vs. ICU) and time (d1 vs. d3). Free piperacillin concentration at days 1 and 3 is lower in ICU than in burn patients (a). There is no correlation between the free and total piperacillin concentration in burn (b, r = −0.104, p = 0.335) and ICU patients (c, r = 0.170, p = 0.206), which is also indicated as slope of the linear regression line did not differ from zero in both collectives
Correlation analyses between eGFR (at d1) and fT>4xMIC (a), fT>1xMIC (b), PIP clearance (c) and TBSA (d) in burn and ICU patients. eGFR values indicating acute kidney injury (AKI) or augmented renal clearance (ARC) are shaded gray. Filled symbols in panel d indicate patients who died while hospitalized
In silico analyses
Considering individual patient parameters, PIP concentrations, PIP protein binding, and a MIC of 16 mg/L, we calculated fT>4xMIC and fT>1xMIC values for 15 different dosing strategies (varying in dosing, dose intervals, and infusion duration) by TDMx (TDMx—model-supported Therapeutic Drug Monitoring for Precision Dosing; Wicha et al. 2015). Individual, simulated PK profiles are exemplarily depicted for one burn patient in Fig. S5 when 4 or 8 g/3 h/qid PIP was to be administered. Computer-based analyses indicate that the best fT>4xMIC and fT>1xMIC values are obtained by the PIP dosage regimen 8 g/3 h/qid (Table 4). When PIP was dosed 4, 6, or 8 g/tid, fT>4xMIC and fT>1xMIC values tended to increase by prolonging the infusion time from 0.5 to 3 h (Table 4). In burn patients, fT>4xMIC following 8 g/3 h/qid was markedly higher than the dosing regimen of 4 g/3 h/qid, which is the maximum approved PIP dosage (Fig. 4b, Table 4). As fT>1xMIC was higher than fT>4xMIC, the difference between 4 g and 8 g/3 h/qid was much lower although two-way ANOVA testing also indicated a slight dosage effect (p = 0.036; Fig. 4a). ICU patients would also benefit from increasing the PIP dosage into the off-label range of 8 g/3 h/qid (Fig. 4). These results were confirmed when absolute numbers of total) of burn patients were counted, reaching 50%fT>MIC, 100%fT>MIC, or 100%fT>4xMIC (Tab. S3).
Predicted fT>1xMIC (a) and fT>4xMIC (b) values (indicating the time (% of 24 h) when the free drug concentration exceeds the 1xMIC and 4xMIC following the PC-based TDMx simulation considering the parameters (sex, body weight, height, serum creatinine, measured PIP concentration, protein binding, and MIC) of burn and ICU patients after infusing 4 g/3 h/qid or 8 g/3 h/qid PIP. Two-way ANOVA testing indicated dosage effects regarding fT>1xMIC (p = 0.036) and fT>4xMIC values (p < 0.0001). A two-way ANOVA was performed to examine the effects of patients (burn vs. ICU) and time (d1 vs. d3)
Off-label use
To assess whether fT>4xMIC and fT>1xMIC values were reached in real life after infusing 8 g/3 h/qid as predicted by our in silico analysis, the ethics committee allowed us to investigate two burn patients using an off-label approach, as the daily total of 32 g (8 g/qid) exceeded the maximum approved dose of 16 g (4 g/qid) PIP. Two patients were included in this program and plasma PIP was determined (Fig. 5a). The eGFR of these two patients was 129 and 109 mL/min, thus not differing from values of burn patients from the observational study (Fig.3). Following off-label treatment using 8 g/3 h/qid PIP, fT>4xMIC was 47.0% and 46.3% and fT>1xMIC was 83.4% and 85.0% for both patients (Fig. 5b). Hence, these values were quite similar to the predicted values of our in silico simulations (Table 4 and Fig. 4). In contrast, fT>4xMIC following the 4 g/3 h/qid regimen was zero while predicted to range between 6 and 18% (Fig. 5c). All other kinetic parameters were similar to those of the main study (Tab. S1). Neither patient showed signs of sepsis. No adverse events were observed.
PIPfree concentration following 8 g/3 h/qid and 4 g/3 h/qid PIP in two burn patients (a). Free PIP concentration was calculated by measuring total PIP concentrations and by considering 13% protein binding as determined for burn patients (see above). The dashed lines indicate the 1xMIC breakpoint (16 mg/L) or 4xMIC breakpoint (64 mg/L) of PIP for P. aeruginosa according to EUCAST (The European Committee on Antimicrobial Susceptibility Testing - EUCAST 2017). fT>1xMIC and fT>4xMIC values following 8 g/3 h/qid (b) or 4 g/3 h/qid PIP (c) which were individually calculated by the web-based TDMx-Software (TDMx - model-supported Therapeutic Drug Monitoring for Precision Dosing; Wicha et al. 2015)). The gray-shaded areas indicate the predicted fT>1xMIC and fT>4xMIC values (means ± SD) as calculated by our simulation approach considering the burn patients of the main study
Discussion
Our study shows that standard doses of intermittently administered PIP are insufficient to achieve therapeutic plasma concentrations greater than one or four times over the MIC by considering a MIC breakpoint for P. aeruginosa of 16 mg/L as defined by EUCAST. Our data suggest that rates of approx. 50%fT>1xMIC or approx. 10%fT>4xMIC do not represent sufficient PK/PD targets in burn patients, which is in accordance with other reports claiming that 100%fT>1xMIC or 40–70%fT>4xMIC are required for PIP as a conservative PK/PD target, respectively (Mohd Hafiz et al. 2012), or even 100%fT>4MIC as a more aggressive indicator (Tam et al. 2002; Tam et al. 2005). The results (approx. 80%fT>1xMIC and 35%fT>4xMIC) for ICU patients were indeed slightly better but certainly not satisfactory.
Blood and organ concentrations that are too low run the risk of inducing therapy failure and/or developing antibiotic resistance (Roberts et al. 2008). Despite knowledge of PK alterations and the therapeutic need for a sufficient antibiotic treatment in burn patients, only few data have been published that focus on the correct dosing of antibiotics in severe burn injuries. Patel and coworkers detected underdosing in up to 60% of burn patients who were treated with six different ß-lactam antibiotics (Patel et al. 2012). Various studies in critically ill patients also confirmed that PIP plasma concentrations might be too low (Sinnollareddy et al. 2012; Taccone et al. 2010; Udy et al. 2012). As one reason for insufficient PIP concentrations, alterations in protein binding were accused of influencing PIP concentrations, as observed in critically ill patients (Wong et al. 2013) and albumin concentration is decreased and plasma protein binding of drugs is altered in burn patients (Blanchet et al. 2008). In the Patel study (Patel et al. 2012), which included only a small number of burn patients (n = 6) receiving PIP treatment, the free PIP concentration was disadvantageously estimated by considering data from healthy volunteers. In contrast, we analyzed not only a markedly larger group of burn patients but also determined that protein binding in these patients is lower than in healthy individuals. However, PPB did not differ between d1 and d3 although plasma albumin slightly increased in this time period as a result of therapeutic supplementation while it was slightly lower (burn 13% and ICU 21%) as reported by others (30%) (Fachinformation Piperacillin/Tazobactam 4 g/0.5 g Pulver zur Herstellung einer Infusionslösung 2015). As PPB was lower, free PIP concentrations might be expected to be higher. However, PIP concentrations were low, which may be attributed to the high volume that was administered within a short time period for fluid resuscitation and a high renal CL. Our observations confirm the conclusion that Vd and CL of PIP are markedly increased in burn patients compared to internal medicine ICU patients, which is in line with the findings of others also showing that these parameters are increased compared to healthy volunteers (Bourget et al. 1996; Bulitta et al. 2007; McManus et al. 1985). An increase in Vd was also attributed to burn-dependent hypoalbuminemia (Udy et al. 2015). As volume was particularly substituted within the first day of hospitalization, which equals d1 of our study and we did not determine PIP plasma concentrations at later time points, we cannot exclude that PIP may be higher later, related to a decrease in CL. Thus, it may be worthwhile to monitor antibiotic concentrations by TDM together with Bayesian forecasting not only at initial therapy but to continue the monitoring in order to maintain the PIP concentrations that were reached by non-burn patients within the therapeutic window. Hence, adverse effects would be expected to only appear at a normal rate by being aware that PIP/TAZ has an excellent safety and tolerability profile (mostly including diarrhea, infused vein reaction, nausea, and headache, most of which were considered mild or moderate) (Gin et al. 2007).
Changes in renal function are particularly important in burn injury and may affect low plasma concentrations. In accordance with others (Conil et al. 2007b; Doh et al. 2010; Jeon et al. 2014), we measured serum creatinine and estimated creatinine clearance (CLCr) by the Cockcroft-Gault formula in burn patients here. Although a linear relationship between CLCr and the Cockcroft-Gault GFR estimation was demonstrated in burn patients, it was shown furthermore that the lowest clearances are overestimated while normal or high CLCr are underestimated (Conil et al. 2007a). Thus, the authors concluded that the Cockcroft-Gault formula is not the best method to assess renal function in burn patients and should be abandoned in favor of direct measurement from a 24-h urine collection. Therefore, this constitutes a limitation of our approach. Nevertheless, we assume that this bias, which is based on eGRF assessment of renal function, will be constant in burn patients, thus still revealing the relative differences between the patients. Acute kidney injury is one of the most important complications and survival decreases in patients with extensive burns covering over 15–20% TBSA (Witkowski et al. 2016). Although the study was not powered to statistically evaluate this issue, we observed a relationship between renal dysfunction and TBSA and that patients died when eGFR was low or decreased within the initial 48 h after injury (Fig. 3d). It seems at least feasible that renal dysfunction will lower PIP clearance, thus increasing drug concentrations inducing toxic effects. Here, standard prescription information for PIP products recommends adjusting PIP dosing and infusion frequency to prevent overdosing and side effects. Although we abstained from adjusting the doses, peak concentrations never reached toxic concentrations. On the other hand, renal blood flow is increased during the hypermetabolic phase, leading to an increased GFR. In our study, 29% of burn patients showed an augmented renal clearance (commonly defined as CLCR ≥ 130 mL min−1), which was causally related to low plasma concentrations not achieving optimal PIP exposures, as also demonstrated by others in critically ill patients who were treated with standard PIP dosing (Sime et al. 2017; Udy et al. 2015). Hence, these authors have suggested that assessing renal function by measuring creatinine clearance can be employed as a useful tool to determine whether achieving the PIP PK/PD target is likely, considering the magnitude of MIC values. We selectively demonstrated in burn patients that eGFR is positively related to PIP clearance while negatively related to %fT>4xMIC, confirming the necessity to monitor renal function to adjust antibiotic treatment. The importance of renal function was taken into consideration by including SCr to optimize PIP dosing by in silico analyses.
As mentioned above, studies in critically ill patients also showed that PIP plasma concentration are too low, allowing the authors to conclude that modified dosing strategies impaired antibiotic therapy (Sinnollareddy et al. 2012; Taccone et al. 2010; Udy et al. 2012). Dose optimization by therapeutic drug monitoring (TDM) is helpful to ensure adequate PIP exposure in critically ill and burn patients (Dellinger et al. 2004; Patel et al. 2012; Roberts et al. 2010). However, there is a great delay for most patients in achieving therapeutic concentrations. As guidelines recommend preventive systemic administration of antibiotics particularly in the perioperative period, limited time is available for TDM-guided PIP adjustment (Yoshino et al. 2016). To overcome this disadvantage in antimicrobial therapy, computer software support is advocated to individualize PIP dosing (Felton et al. 2014). In this context, Monte Carlo simulations were demonstrated to provide adequate PIP exposure for the management of febrile neutropenia (Sime et al. 2017). To estimate optimal PIP dosing, we used the TDMx platform, which is an attractive, state-of-the-art, pharmacometric, open access software tool (TDMx—model-supported Therapeutic Drug Monitoring for Precision Dosing; Wicha et al. 2015). By using this approach, we could simulate different approaches for each patient by varying the dosage and infusion durations and also including the comparison between continuous versus intermittent infusions. This comparison might be important as conflicting results have been published. A Cochrane analysis on antibiotic treatment of severe acute infections demonstrated no differences in mortality, infection recurrence, clinical cure, superinfection post-therapy, and safety outcomes when comparing continuous infusions of intravenous antibiotics to traditional, intermittent infusions of antibiotics (Shiu et al. 2013) while a very recent study showed that, compared to intermittent dosing, administration of β-lactam antibiotics by continuous infusion in critically ill patients with severe sepsis was associated with decreased hospital mortality (Roberts et al. 2016). In our in silico analyses, we showed that increasing both the dose and the infusion time resulted in greater fT>1xMIC and fT>4xMIC values. Similar findings were observed in patients treated with meropenem for ventilator-associated pneumonia after administration as 3-h infusion or bolus injection (Jaruratanasirikul et al. 2005). Extended infusion regimens of β-lactams were found to be the best option to ensure optimal protection against the most susceptible isolates in adult patients with cystic fibrosis (Butterfield et al. 2014) and sepsis (Vardakas et al. 2018). Based also on the observation that commonly employed, intermittent PIP doses (4.0 g tid or qid) are highly likely to result in subtherapeutic exposures, dosing simulations for optimal PIP exposure revealed that prolonged infusion regimens are necessary to cover common pathogens isolated from patients with febrile neutropenia (E. coli, K. pneumoniae, and P. aeruginosa (Sime et al. 2017). We detected that continuous infusions of 12 or 16 g PIP sid produced good target effects for fT>1xMIC. However, fT>4xMIC values remained extremely low. As 8 g/3 h/qid PIP promised the best results in TDMx dosing simulation, we proposed this PIP dosing regimen for the treatment of burn patients knowing that this dosage is off-label. To test whether the prediction is true in reality, we treated two burn patients according to this PIP regimen and compared it with the maximum approved dosing regimen of 4 g/3 h/qid. Both approaches confirmed the predicted fT>1xMIC and fT>4xMIC values (Fig. 5). It additionally became evident that the conservative or aggressive target (100%fT>1xMIC or 40–70%fT>4xMIC) (Taccone et al. 2010) was almost reached by off-label use of PIP while the maximum approved dosing regimen failed. In contrast, the stricter 100%fT>4xMIC target (Tam et al. 2002; Tam et al. 2005) was not fulfilled by 8 g/3 h/qid PIP. Side effects and, particularly, seizures were not observed; however, patients were only treated for 1 day with the high PIP dose and they were sedated due to the burn injury.
We determined that the following aspects limit the importance of our study: (i) we only estimated GFR according to the Cockroft-Gault equation and did not measure creatinine clearance, being aware that Cockroft-Gault estimation may be suboptimal to assess renal function in burn patients (Conil et al. 2007a); (ii) we used the clinical breakpoint defined by EUCAST for P. aeruginosa as the target MIC instead of intraindividual MIC values of the patient, who is also influenced by local conditions; (iii) as no differences were seen between the 2 days, it may be concluded that volume displacement either has no effect on PIP concentration or that the gap between the two measurements was too close. Thus, it may be necessary to additionally determine PIP concentrations at later time points in a follow-up trial; and (iv) this study was not designed to evaluate clinical outcomes concerning mortality, infection prevention or recurrence, clinical cure, superinfection post-therapy, and safety outcomes. To obtain such results, it would be necessary to perform a follow-up (multicenter) study based on a larger cohort of burn patients who would be treated as suggested with high-dose PIP and prolonged infusion duration.
Conclusions
Our results clearly show that the standard dosing regimen by infusing 4 g/0.5/tid PIP is unlikely to achieve optimal PIP exposure in burn or in critically ill patients from the ICU. This is mainly driven by an increase in PIP clearance. In silico dosing simulation predicted that it may be necessary to increase PIP doses (8 g/qid) and to prolong infusion duration (3 h) in burn patients to achieve higher blood concentrations of the drug. As eGFR were found by using Bayesian pharmacokinetic analysis to correlate with PIP concentration in burn patients, we aim to modify TDMx to better integrate this parameter in in silico simulations to better predict PIP dosing for each burn patient prior to starting PIP therapy. We additionally intend in a follow-up project to perform population pharmacokinetic analyses that will likely identify further co-variates, which then may improve the PIP dosing in burn and ICU patients.
Abbreviations
- ABSI:
-
Abbreviated Burn Severity Risk Index
- AKI:
-
Acute kidney injury
- ARC:
-
Augmented renal clearance
- CL:
-
Clearance
- CLCr:
-
Creatinine clearance
- D:
-
Day
- ICU:
-
Intensive care unit
- MIC:
-
Minimal inhibition concentration
- PIP:
-
Piperacillin
- PK:
-
Pharmacokinetic
- SAPS II score:
-
Simplified Acute Physiology Score
- SCr:
-
Serum creatinine
- t 1/2 :
-
Half-life time
- TAZ:
-
Tazobactam
- TBSA:
-
Total burn surface area
- TDM:
-
Therapeutic drug monitoring
- TISS:
-
Therapeutic Intervention Scoring System
- Vd :
-
Volume of distribution
- PBB:
-
Plasma protein binding
- tid:
-
Three times a day
- qid:
-
Four times a day
- sid:
-
Once a day
References
Alharbi Z, Piatkowski A, Dembinski R, Reckort S, Grieb G, Kauczok J, Pallua N (2012) Treatment of burns in the first 24 hours: simple and practical guide by answering 10 questions in a step-by-step form. World J Emerg Surg 7:13. https://doi.org/10.1186/1749-7922-7-13
Avni T, Levcovich A, Ad-El DD, Leibovici L, Paul M (2010) Prophylactic antibiotics for burns patients: systematic review and meta-analysis. BMJ 340:c241. https://doi.org/10.1136/bmj.c241
Barajas-Nava LA, López-Alcalde J, Roqué i Figuls M, Solà I, Bonfill Cosp X (2013) Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst Rev CD008738. https://doi.org/10.1002/14651858.CD008738.pub2
Bergan T (1981) Overview of acylureidopenicillin pharmacokinetics. Scand J Infect Dis Suppl 29:33–48
Blanchet B, Jullien V, Vinsonneau C, Tod M (2008) Influence of burns on pharmacokinetics and pharmacodynamics of drugs used in the care of burn patients. Clin Pharmacokinet 47:635–654. https://doi.org/10.2165/00003088-200847100-00002
Bourget P, Lesne-Hulin A, Le Reveillé R, Le Bever H, Carsin H (1996) Clinical pharmacokinetics of piperacillin-tazobactam combination in patients with major burns and signs of infection. Antimicrob Agents Chemother 40:139–145
Briscoe SE, McWhinney BC, Lipman J, Roberts JA, Ungerer JPJ (2012) A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 907:178–184. https://doi.org/10.1016/j.jchromb.2012.09.016
Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, Sörgel F (2007) Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 51:2497–2507. https://doi.org/10.1128/AAC.01477-06
Butterfield JM, Lodise TP, Beegle S, Rosen J, Farkas J, Pai MP (2014) Pharmacokinetics and pharmacodynamics of extended-infusion piperacillin/tazobactam in adult patients with cystic fibrosis-related acute pulmonary exacerbations. J Antimicrob Chemother 69:176–179. https://doi.org/10.1093/jac/dkt300
Cartotto R, Callum J (2012) A review of the use of human albumin in burn patients. J Burn Care Res 33:702–717. https://doi.org/10.1097/BCR.0b013e31825b1cf6
Chim H, Tan BH, Song C (2007) Five-year review of infections in a burn intensive care unit: high incidence of Acinetobacter baumannii in a tropical climate. Burns 33:1008–1014. https://doi.org/10.1016/j.burns.2007.03.003
Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clin Microbiol Rev 19:403–434. https://doi.org/10.1128/CMR.19.2.403-434.2006
Conil JM, Georges B, Fourcade O, Seguin T, Lavit M, Samii K, Houin G, Tack I, Saivin S (2007a) Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol 63:583–594. https://doi.org/10.1111/j.1365-2125.2006.02807.x
Conil JM, Georges B, Lavit M, Laguerre J, Samii K, Houin G, Saivin S (2007b) A population pharmacokinetic approach to ceftazidime use in burn patients: influence of glomerular filtration, gender and mechanical ventilation. Br J Clin Pharmacol 64:27–35. https://doi.org/10.1111/j.1365-2125.2007.02857.x
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10 quiz 11-12
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM, Surviving Sepsis Campaign Management Guidelines Committee (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Demling RH (2005) The burn edema process: current concepts. J Burn Care Rehabil 26:207–227
Doh K, Woo H, Hur J, Yim H, Kim J, Chae H, Han S, Yim DS (2010) Population pharmacokinetics of meropenem in burn patients. J Antimicrob Chemother 65:2428–2435. https://doi.org/10.1093/jac/dkq317
Fachinformation Piperacillin/Tazobactam 4 g / 0,5 g Pulver zur Herstellung einer Infusionslösung (2015). https://wwwfachinfode/suche/fi/013311. Assessed August 2015
Felton TW, Roberts JA, Lodise TP, Guilder MV, Boselli E, Neely MN, Hope WW (2014) Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob Agents Chemother 58:4094–4102. https://doi.org/10.1128/AAC.02664-14
Gin A, Dilay L, Karlowsky JA, Walkty A, Rubinstein E, Zhanel GG (2007) Piperacillin-tazobactam: a beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti-Infect Ther 5:365–383. https://doi.org/10.1586/14787210.5.3.365
Guilabert P, Usúa G, Martín N, Abarca L, Barret JP, Colomina MJ (2016) Fluid resuscitation management in patients with burns: update. Br J Anaesth 117:284–296. https://doi.org/10.1093/bja/aew266
Jaruratanasirikul S, Sriwiriyajan S, Punyo J (2005) Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob Agents Chemother 49:1337–1339. https://doi.org/10.1128/AAC.49.4.1337-1339.2005
Jeon S, Han S, Lee J, Hong T, Paek J, Woo H, Yim D-S (2014) Population pharmacokinetic analysis of piperacillin in burn patients. Antimicrob Agents Chemother 58:3744–3751. https://doi.org/10.1128/AAC.02089-13
Li C, Du X, Kuti JL, Nicolau DP (2007) Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. https://doi.org/10.1128/AAC.00294-06
Mann EA, Baun MM, Meininger JC, Wade CE (2012) Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock 37:4–16. https://doi.org/10.1097/SHK.0b013e318237d6bf
McKinnon PS, Paladino JA, Schentag JJ (2008) Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. https://doi.org/10.1016/j.ijantimicag.2007.12.009
McManus AT, Mason AD, McManus WF, Pruitt BA (1985) Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol 4:219–223
McWhinney BC, Wallis SC, Hillister T, Roberts JA, Lipman J, Ungerer JPJ (2010) Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 878:2039–2043. https://doi.org/10.1016/j.jchromb.2010.05.027
Mir MA, Khurram MF, Khan AH (2017) What should be the antibiotic prescription protocol for burn patients admitted in the department of burns, plastic and reconstructive surgery. Int Wound J 14:194–197. https://doi.org/10.1111/iwj.12588
Mohd Hafiz A-A, Staatz CE, Kirkpatrick CMJ, Lipman J, Roberts JA (2012) Continuous infusion vs. bolus dosing: implications for beta-lactam antibiotics. Minerva Anestesiol 78:94–104
Patel BM, Paratz J, See NC, Muller MJ, Rudd M, Paterson D, Briscoe SE, Ungerer J, McWhinney BC, Lipman J, Roberts JA (2012) Therapeutic drug monitoring of beta-lactam antibiotics in burns patients--a one-year prospective study. Ther Drug Monit 34:160–164. https://doi.org/10.1097/FTD.0b013e31824981a6
Rafla K, Tredget EE (2011) Infection control in the burn unit. Burns 37:5–15. https://doi.org/10.1016/j.burns.2009.06.198
Roberts JA (2011) Using PK/PD to optimize antibiotic dosing for critically ill patients. Curr Pharm Biotechnol 12:2070–2079
Roberts JA, Kruger P, Paterson DL, Lipman J (2008) Antibiotic resistance--what’s dosing got to do with it? Crit Care Med 36:2433–2440. https://doi.org/10.1097/CCM.0b013e318180fe62
Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J (2010) Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents 36:332–339. https://doi.org/10.1016/j.ijantimicag.2010.06.008
Roberts JA, Paul SK, Akova M, Bassetti M, de Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, Margarit Ribas A, de Waele JJ, de Crop L, Spapen H, Wauters J, Dugernier T, Jorens P, Dapper I, de Backer D, Taccone FS, Rello J, Ruano L, Afonso E, Alvarez-Lerma F, Gracia-Arnillas MP, Fernandez F, Feijoo N, Bardolet N, Rovira A, Garro P, Colon D, Castillo C, Fernado J, Lopez MJ, Fernandez JL, Arribas AM, Teja JL, Ots E, Carlos Montejo J, Catalan M, Prieto I, Gonzalo G, Galvan B, Blasco MA, Meyer E, del Nogal F, Vidaur L, Sebastian R, Garde PM, Martin Velasco MM, Zaragoza Crespo R, Esperatti M, Torres A, Montravers P, Baldesi O, Dupont H, Mahjoub Y, Lasocki S, Constantin JM, Payen JF, Martin C, Albanese J, Malledant Y, Pottecher J, Lefrant JY, Jaber S, Joannes-Boyau O, Orban C, Ostermann M, McKenzie C, Berry W, Smith J, Lei K, Rubulotta F, Gordon A, Brett S, Stotz M, Templeton M, Rhodes A, Ebm C, Moran C, Kaukonen KM, Pettila V, Dimopoulos G, Koulenti D, Xristodoulou A, Theodorou V, Kouliatsis G, Sertaridou E, Anthopoulos G, Choutas G, Rantis T, Karatzas S, Balla M, Papanikolaou M, Myrianthefs P, Gavala A, Fildisis G, Koutsoukou A, Kyriakopoulou M, Petrochilou K, Kompoti M, Michalia M, Clouva-Molyvdas FM, Gkiokas G, Nikolakopoulos F, Psychogiou V, Malliotakis P, Akoumianaki E, Lilitsis E, Koulouras V, Nakos G, Kalogirou M, Komnos A, Zafeiridis T, Chaintoutis C, Arvaniti K, Matamis D, Chaintoutis C, Kydona C, Gritsi-Gerogianni N, Giasnetsova T, Giannakou M, Soultati I, Chytas I, Antoniadou E, Antipa E, Lathyris D, Koukoubani T, Paraforou T, Spiropoulou K, Bekos V, Spring A, Kalatzi T, Nikolaou H, Laskou M, Strouvalis I, Aloizos S, Kapogiannis S, Soldatou O, Bassetti M, Adembri C, Villa G, Giarratano A, Maurizio Raineri S, Cortegiani A, Montalto F, Strano MT, Ranieri VM, Sandroni C, de Pascale G, Molin A, Pelosi P, Montagnani L, Urbino R, Mastromauro I, de Rosa FG, Ranieri VM, Cardoso T, Afonso S, Goncalves-Pereira J, Baptista JP, Akova M, Ozveren A (2014) DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. https://doi.org/10.1093/cid/ciu027
Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J (2016) Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 194:681–691. https://doi.org/10.1164/rccm.201601-0024OC
Schleibinger M, Steinbach CL, Töpper C, Kratzer A, Liebchen U, Kees F, Salzberger B, Kees MG (2015) Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 80:525–533. https://doi.org/10.1111/bcp.12636
Shiu J, Wang E, Tejani AM, Wasdell M (2013) Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst Rev CD008481. https://doi.org/10.1002/14651858.CD008481.pub2
Shorr AF (2009) Review of studies of the impact on gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med 37:1463–1469. https://doi.org/10.1097/CCM.0b013e31819ced02
Sime FB et al (2017) Are standard doses of piperacillin in piperacillin/tazobactam regimens adequate for the management of febrile neutropenia? Answers from population pharmacokinetic modelling and Monte Carlo simulations. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00311-17
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315:801–810. https://doi.org/10.1001/jama.2016.0287
Sinnollareddy MG, Roberts MS, Lipman J, Roberts JA (2012) β-Lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: a structured review. Clin Exp Pharmacol Physiol 39:489–496. https://doi.org/10.1111/j.1440-1681.2012.05715.x
Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Witebolle X, de Backer D, Layeux B, Wallemacq P, Vincent JL, Jacobs F (2010) Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14:R126. https://doi.org/10.1186/cc9091
Tagami T, Matsui H, Fushimi K, Yasunaga H (2016) Prophylactic antibiotics may improve outcome in patients with severe burns requiring mechanical ventilation: propensity score analysis of a Japanese Nationwide Database. Clin Infect Dis 62:60–66. https://doi.org/10.1093/cid/civ763
Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL (2002) Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 50:425–428
Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA (2005) Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:4920–4927. https://doi.org/10.1128/AAC.49.12.4920-4927.2005
TDMx - model-supported Therapeutic Drug Monitoring for Precision Dosing (2017). http://www.tdmx.eu/. Assessed Nov 2017
The European Committee on Antimicrobial Susceptibility Testing - EUCAST (2017). http://www.eucast.org/clinical_breakpoints/. Assessed Oktober 2017
Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S (2004) Pseudomonas infections in the thermally injured patient. Burns 30:3–26
Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, Lipman J, Roberts JA (2012) Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 142:30–39. https://doi.org/10.1378/chest.11-1671
Udy AA, Lipman J, Jarrett P, Klein K, Wallis SC, Patel K, Kirkpatrick C, Kruger PS, Paterson DL, Roberts MS, Roberts JA (2015) Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care 19:28. https://doi.org/10.1186/s13054-015-0750-y
Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME (2018) Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis 18:108–120. https://doi.org/10.1016/S1473-3099(17)30615-1
Weinbren MJ (1999) Pharmacokinetics of antibiotics in burn patients. J Antimicrob Chemother 44:319–327. https://doi.org/10.1093/jac/44.3.319
Wicha SG, Kees MG, Solms A, Minichmayr IK, Kratzer A, Kloft C (2015) TDMx: a novel web-based open-access support tool for optimising antimicrobial dosing regimens in clinical routine. Int J Antimicrob Agents 45:442–444. https://doi.org/10.1016/j.ijantimicag.2014.12.010
Witkowski W, Kawecki M, Surowiecka-Pastewka A, Klimm W, Szamotulska K, Niemczyk S (2016) Early and late acute kidney injury in severely burned patients. Med Sci Monit 22:3755–3763
Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA (2013) Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 57:6165–6170. https://doi.org/10.1128/AAC.00951-13
Yoshino Y, Ohtsuka M, Kawaguchi M, Sakai K, Hashimoto A, Hayashi M, Madokoro N, Asano Y, Abe M, Ishii T, Isei T, Ito T, Inoue Y, Imafuku S, Irisawa R, Ohtsuka M, Ogawa F, Kadono T, Kawakami T, Kukino R, Kono T, Kodera M, Takahara M, Tanioka M, Nakanishi T, Nakamura Y, Hasegawa M, Fujimoto M, Fujiwara H, Maekawa T, Matsuo K, Yamasaki O, le Pavoux A, Tachibana T, Ihn H, The Wound/Burn Guidelines Committee (2016) The wound/burn guidelines - 6: guidelines for the management of burns. J Dermatol 43:989–1010. https://doi.org/10.1111/1346-8138.13288
Acknowledgments
The authors gratefully acknowledge Prof. Dr. Frieder Kees (Institute of Pharmacology, University of Regensburg) for his support in establishing HPLC analysis, Dr. Reinhard Vonthein (Institute of Medical Biometry and Statistics, University of Lübeck) for assistance in power analysis, Ines Stölting for assistance in HPLC analysis, and Sherryl Sundell for improving the English style.
Funding
The study was funded by the Department of Pharmacy of the University Hospital Schleswig-Holstein, Campus Lübeck, Lübeck, Germany.
Author information
Authors and Affiliations
Contributions
KO, TK, EK, JT, PM, and WR conceived the study. KO, JT, TK, EK, PM, TG, and WR were involved in protocol development, ethical approval, and implementation. KO, TK, TG, and WR collected the data. KO and WR completed the laboratory analysis and quality assurance. KO, SW, and WR performed the statistical and pharmacokinetic analyses. KO and WR wrote the manuscript draft, and all of the remaining authors contributed to subsequent revisions. WR takes responsibility for archiving the data and guarantees the integrity of the paper from inception to publication. All of the authors have read and approved the article for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the University (reference number 13-275).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 183 kb)
Rights and permissions
About this article
Cite this article
Olbrisch, K., Kisch, T., Thern, J. et al. After standard dosage of piperacillin plasma concentrations of drug are subtherapeutic in burn patients. Naunyn-Schmiedeberg's Arch Pharmacol 392, 229–241 (2019). https://doi.org/10.1007/s00210-018-1573-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1573-6