Abstract
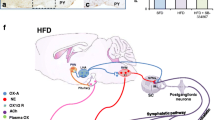
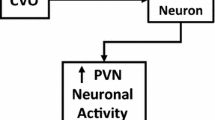
Orexins (orexin A and orexin B), neuropeptides of hypothalamic origin also known as hypocretins, have been well documented for regulating the different physiological functions including feeding, sleep wakefulness, stress, and reward. However, from the past few years, orexins have evolved as an emerging biomarker for various endocrine disorders including diabetes mellitus and obesity which ultimately leads to various cardiovascular risk factors. Orexins exist in two isoforms orexin A and orexin B and exert their effect by acting on the G protein-coupled receptors orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R). Furthermore, localization of orexinergic neurons in the different brain regions has been involved in regulating the cardiovascular and sympathetic activity. Growing evidences have addressed the potential role of orexins including orexin A and orexin B in modulating the hypertension via exerting their effect on the mean arterial pressure (MAP), heart rate (HR), and renal sympathetic nerve activity (RSNA). The present review summarizes the central role orexins in the hypertension along with the possible mechanism.

Similar content being viewed by others
References
Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ (2001) Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281:R1801–R1807
Beig MI, Horiuchi J, Dampney RA, Carrive P (2015) Both Ox1R and Ox2R orexin receptors contribute to the cardiorespiratory response evoked from the perifornical hypothalamus. Clin Exp Pharmacol Physiol 42(10):1059–1067
Burdakov D, Karnani MM, Gonzalez A (2013) Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav 121:117–124
Chan SH, Wang LL, Wang SH, Chan JY (2001) Differential cardiovascular responses to blockade of nNOS or iNOS in rostral ventrolateral medulla of the rat. Br J Pharmacol 133(4):606–614
Chang AY, Chan JY, Chan SH (2003) Differential distribution of nitric oxide synthase isoforms in the rostral ventrolateral medulla of the rat. J Biomed Sci 10(3):285–291
Chen CT, Hwang LL, Chang JK, Dun NJ (2000) Pressure effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Phys Regul Integr Comp Phys 278(3):692–697
Cheng SB, Kuchiiwa S, Gao HZ, Kuchiiwa T, Nakagawa S (2003) Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci Res 46(1):53–62
Ciriello J, Caverson MM, McMurray JC, Bruckschwaiger EB (2013) Co-localization of hypocretin-1 and leucine-enkephalin in hypothalamic neurons projecting to the nucleus of the solitary tract and their effect on arterial pressure. Neuroscience 250:599–613
Clifford L, Dampney BW, Carrive P (2015) Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Exp Physiol 100(4):388–398
Cluderay JE, Harrison DC, Hervieu GJ (2002) Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept 104(1–3):131–144
D'Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW (2006) Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressure recovery in obese Zucker rats. Hypertension 48(6):1109–1115
Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A 96(2):748–753
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95(1):322–327
Guo ZL, Tjen-A-Looi SC, Fu LW, Longhurst JC (2009) Nitric oxide in rostral ventrolateral medulla regulates cardiac-sympathetic reflexes: role of synthase isoforms. Am J Physiol Heart Circ Physiol 297(4):H1478–H1486
Guo BQ, Jia M, Liu JX, Zhang Z (2010) Cardiovascular effect of intracerebroventricular injection of orexin-1 receptor antagonist in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 26(3):278–283
Guyenet PG, Stornetta RL (2004) The presympathetic cells of the rostral ventrolateral medulla (RVLM): anatomy, physiology and role in the control of circulation. In: Dun NJ, Machado BH, Pilowsky PM (eds) Neural mechanisms of cardiovascular regulation. Kluwer Academic, Norwell, MA, pp 187–218
Hamid SA, Totzeck M, Drexhage C, Thompson I, Fowkes RC, Rassaf T, Baxter GF (2010) Nitric oxide/cGMP signalling mediates the cardioprotective action of adrenomedullin in reperfused myocardium. Basic Res Cardiol 105(2):257–266
Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL (2010) Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther 334(2):522–529
Ibrahim BM, Abdel-Rahman AA (2012) Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressure response in conscious rats. J Pharmacol Exp Ther 341(3):579–586
Inutsuka A, Yamanaka A (2013) The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne) 4:18
Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P, Head GA (2016) Contribution of orexin to the neurogenic hypertension in BPH/2J mice. Hypertension 67(5):959–969
Jiang MY, Chen J, Wang J, Xiao F, Zhang HH, Zhang CR, Du DS, Cao YX, Shen LL, Zhu DN (2011) Nitric oxide modulates cardiovascular function in the rat by activating adenosine A2A receptors and inhibiting acetylcholine release in the rostral ventrolateral medulla. Clin Exp Pharmacol Physiol 38(6):380–386
Kannan H, Shirasaka T, Watanabe S, Yu NS, Kuitake T, Takasaki M (2007) Central action of orexins on sympathetic outflow and cardiovascular function with a focus on the paraventricular nucleus of the hypothalamus. Masui 56(1):30–39
Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T (2003) Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 285(3):581–593
Kimura Y, Hirooka Y, Sagara Y, Ito K, Kishi T, Shimokawa H, Takeshita A, Sunagawa K (2005) Overexpression of inducible nitric oxide synthase in rostral ventrolateral medulla causes hypertension and sympathoexcitation via an increase in oxidative stress. Circ Res 96(2):252–260
Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C (2012) Brain orexin promotes obesity resistance. Ann N Y Acad Sci 1264:72–86
Lee YH, Dai YW, Huang SC, Li TL, Hwang LL (2013) Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol 98(7):1145–1155
Lee YH, Tsai MC, Li TL, Dai YW, Huang SC, Hwang LL (2015) Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla. Exp Physiol 100(9):993–1007
Li A, Hindmarch CC, Nattie EE, Paton JF (2013) Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 591(17):4237–4248
Lin Y, Matsumura K, Tsuchihashi T, Abe I, Iida M (2002) Chronic central infusion of orexin-a increases arterial pressure in rats. Brain Res Bull 57(5):619–622
Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ (2002) Pressure response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104(1–3):75–81
Machado NL, Silva FC, Chianca DA Jr, de Menezes RC (2016) Nitric oxide modulates blood pressure through NMDA receptors in the rostral ventrolateral medulla of conscious rats. Brain Res 1643:159–167
Martins-Pinge MC, Garcia MR, Zoccal DB, Crestani CC, Pinge-Filho P (2007) Differential influence of iNOS and nNOS inhibitors on rostral ventrolateral medullary mediated cardiovascular control in conscious rats. Auton Neurosci 131(1–2):65–69
Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM (2013) Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 9(3):490
Matsumura K, Tsuchihashi T, Abe I (2001) Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37(6):1382–1387
Mischel NA, Subramanian M, Dombrowski MD, Llewellyn-Smith IJ, Mueller PJ (2015) (In) activity-Related Neuroplasticity in Brainstem Control of Sympathetic Outflow: Unraveling Underlying Molecular, Cellular and Anatomical Mechanisms. Am J Phys Heart Circ Phys ajpheart-00929
Morgan DA, Anderson EA, Mark AL (1995) Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension 25:834–838
Murphy MN, Mizuno M, Downey RM, Squiers JJ, Squiers KE, Smith SA (2013) Neuronal nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle reflexes in hypertensive rats. Am J Physiol Heart Circ Physiol 304(11):1547–1557
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18(23):9996–10015
Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ (1984) Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol 228(2):168–185
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92(5):1
Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC (1999) Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 831(1–2):248–253
Schwimmer H, Stauss HM, Abboud F, Nishino S, Mignot E, Zeitzer JM (2010) Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J Appl Physiol 109(4):1053–1063
Shahid IZ, Rahman AA, Pilowsky PM (2011) Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol 162(4):961–973
Shahid IZ, Rahman AA, Pilowsky PM (2012) Orexin A in rat rostral ventrolateral medulla is pressure, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol 165(7):2292–2303
Shih CD, Chuang YC (2007) Nitric oxide and GABA mediate bi-directional cardiovascular effects of orexin in the nucleus tractus solitarii of rats. Neuroscience 149(3):625–635
Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H (1999) Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol 277(6):R1780–R1785
Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H (2001) Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Phys Regul Integr Comp Phys 281(4):1114–1118
Silvani A, Bastianini S, Berteotti C, Lo Martire V, Zoccoli G (2013) Treating hypertension by targeting orexin receptors: potential effects on the sleep-related blood pressure dipping profile. J Physiol 591(23):6115–6116
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438(1–2):71–75
Wu WC, Wang Y, Su CK, Chai CY (2001) The nNOS/cGMP signal transducing system is involved in the cardiovascular responses induced by activation of NMDA receptors in the rostral ventrolateral medulla of cats. Neurosci Lett 310(2–3):121–124
Xiao F, Jiang M, Du D, Xia C, Wang J, Cao Y, Shen L, Zhu D (2013) Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology 67:16–24
Yang B, Samson WK, Ferguson AV (2003) Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J Neurosci 23(15):6215–6222
Zhang W, Fukuda Y, Kuwaki T (2005) Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett 385(2):131–136
Zhou JJ, Yuan F, Zhang Y, Li DP (2015) Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology 99:481–490
Acknowledgements
The funding has been provided by the Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rani, M., Kumar, R. & Krishan, P. Implicating the potential role of orexin in hypertension. Naunyn-Schmiedeberg's Arch Pharmacol 390, 667–676 (2017). https://doi.org/10.1007/s00210-017-1378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1378-z