Abstract
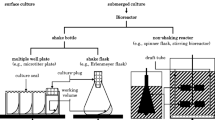
Shake-flask culture, an aerobic submerged culture, has been used in various applications involving cell cultivation. However, it is not designed for forced aeration. Hence, this study aimed to develop a small-scale submerged shaking culture system enabling forced aeration into the medium. A forced aeration control system for multiple vessels allows shaking, suppresses volatilization, and is attachable externally to existing shaking tables. Using a specially developed plug, medium volatilization was reduced to less than 10%, even after 45 h of continuous aeration (~ 60 mL/min of dry air) in a 50 mL working volume. Escherichia coli IFO3301 cultivation with aeration was completed within a shorter period than that without aeration, with a 35% reduction in the time-to-reach maximum bacterial concentration (26.5 g-dry cell/L) and a 1.25-fold increase in maximum concentration. The maximum bacterial concentration achieved with aeration was identical to that obtained using the Erlenmeyer flask, with a 65% reduction in the time required to reach it.



Similar content being viewed by others
References
Anderlei T, Zang W, Papaspyrou M, Büchs J (2004) Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochem Eng J 17:187–194
Azizan A, Büchs J (2017) Three-dimensional (3D) evaluation of liquid distribution in shake flask using an optical fluorescence technique. J Biol Eng 11:28
Dinter C, Gumprecht A, Menze MA, Azizan A, Niehoff PJ, Hansen S, Büchs J (2024) Validation of computational fluid dynamics of shake flask experiments at moderate viscosity by liquid distributions and volumetric power inputs. Sci Rep 14:3658
Falch EA, Hedén CG (1963) Disposable shaker flasks. Biotechnol Bioeng 5:211–220
Ganjave SD, Dodia H, Sunder AV, Madhu S, Wangikar PP (2022) High cell density cultivation of E coli in shake flasks for the production of recombinant proteins. Biotechnol Rep (amst) 33:e00694
Hansen S, Kensy F, Käser A, Büchs J (2011) Potential errors in conventional DOT measurement techniques in shake flasks and verification using a rotating flexitube optical sensor. BMC Biotechnol 11:49
Hansen S, Hariskos I, Luchterhand B, Büchs J (2012) Development of a modified respiration activity monitoring system for accurate and highly resolved measurement of respiration activity in shake flask fermentations. J Biol Eng 6:11
Hansen S, Gumprecht A, Micheel L, Hennemann HG, Enzmann F, Blümke W (2022) Implementation of perforated concentric ring walls considerably improves gas-liquid mass transfer of shaken bioreactors. Front Bioeng Biotechnol 10:894295
Hara M (1965) Free-surface observations of various liquid in a rotary shaken flask (i) some analysis and experiment, assuming liquid as ideal. J Ferment Tech 43:590–596
Hermann R, Walther N, Maier U, Büchs J (2001) Optical method for the determination of the oxygen-transfer capacity of small bioreactors based on sulfite oxidation. Biotechnol Bioeng 74:355–363
Jeude M, Dittrich B, Niederschulte H, Anderlei T, Knocke C, Klee D, Büchs J (2006) Fed-batch mode in shake flasks by slow-release technique. Biotechnol Bioeng 95:433–445
Kato I, Tanaka H (1998) Development of a novel box-shaped shake flask with efficient gas exchange capacity. J Ferment Bioeng 85:404–409
Kluyver A, Perquin L (1933) Zur methodik der schimmel-stoffwechseluntersuchung. Biochem Z 266:68–81 (in German)
Maier U, Losen M, Büchs J (2004) Advances in understanding and modeling the gas–liquid mass transfer in shake flasks. Biochem Eng J 17:155–167
Palacios-Morales C, Aguayo-Vallejo JP, Trujillo-Roldán MA, Zenit R, Ascanio G, Córdova-Aguilar MS (2016) The flow inside shaking flasks and its implication for mycelial cultures. Chem Eng Sci 152:163–171
Panula-Perälä J, Šiurkus J, Vasala A, Wilmanowski R, Casteleijn MG, Neubauer P (2008) Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks. Microb Cell Fact 7:31
Philip P, Meier K, Kern D, Goldmanns J, Stockmeier F, Bähr C, Büchs J (2017) Systematic evaluation of characteristics of the membrane-based fed-batch shake flask. Microb Cell Fact 16:122
Reynoso-Cereceda GI, Garcia-Cabrera RI, Valdez-Cruz NA, Trujillo-Roldán MA (2016) Shaken flasks by resonant acoustic mixing versus orbital mixing: mass transfer coefficient kLa characterization and Escherichia coli cultures comparison. Biochem Eng J 105:379–390
Schulte A, Schilling JV, Nolten J, Korona A, Krömke H, Vennekotter JB, Schillheim B, Wessling M, Conrath U, Büchs J (2018) Parallel online determination of ethylene release rate by shaken parsley cell cultures using a modified RAMOS device. BMC Plant Biol 18:101
Takahashi M, Aoyagi H (2018a) Effect of intermittent opening of breathable culture plugs and aeration of headspace on the structure of microbial communities in shake-flask culture. J Biosci Bioeng 126:96–101
Takahashi M, Aoyagi H (2018b) Practices of shake-flask culture and advances in monitoring CO2 and O2. Appl Microbiol Biotechnol 102:4279–4289
Takahashi M, Aoyagi H (2018c) Monitoring of CO2 and O2 concentrations in the headspace of Sakaguchi flasks during liquid culture of microorganism. Appl Microbiol Biotechnol 102:6637–6645
Takahashi M, Aoyagi H (2020a) Analysis and effect of conventional flasks in shaking culture of Escherichia coli. AMB Express 10:77
Takahashi M, Aoyagi H (2020b) Analysis of porous breathable stopper and development of PID control for gas phase during shake-flask culture with microorganisms. Appl Microbiol Biotechnol 104:8925–8936
Takahashi M, Aoyagi H (2021) Development of a bellows pumping device for enhancing ventilation to shake-flask systems. Biochem Eng J 174:108098
Takahashi M, Aoyagi H (2022) Control of carbon dioxide concentration in headspace of multiple flasks using both non-electric bellows pump and shaking incubator. J Biosci Bioeng 134:240–247
Takahashi M, Sawada Y, Aoyagi H (2017) Development of a circulation direct sampling and monitoring system for O2 and CO2 concentrations in the gas–liquid phases of shake-flask systems during microbial cell culture. AMB Express 7:163
Takahashi M, Honzawa T, Tominaga R, Aoyagi H (2020) Analysis of the influence of flame sterilization included in sampling operations on shake-flask cultures of microorganisms. Sci Rep 10:10385
Takahashi M, Kato T, Aoyagi H (2023) Development of a feed-forward control system for medium in shake-flask culture. Biochem Eng J 196:108939
Ude C, Hentrop T, Lindner P, Lücking TM, Scheper T, Beutel S (2015) New perspectives in shake flask pH control using a 3D-printed control unit based on pH online measurement. Sens Actuators B Chem 221:1035–1043
Yang T, Huang Y, Han Z, Liu H, Zhang R, Xu Y (2013) Novel disposable flexible bioreactor for Escherichia coli culture in orbital shaking incubator. J Biosci Bioeng 116:452–459
Zhang H, Williams-Dalson W, Keshavarz-Moore E, Shamlou PA (2005) Computational-fluid-dynamics (CFD) analysis of mixing and gas-liquid mass transfer in shake flasks. Biotechnol Appl Biochem 41:1–8
Acknowledgements
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language. The authors would like to thank Masahiko Kanagawa in the Institute of Applied Biochemistry, University of Tsukuba for technical assistance in the preliminary stages of this work.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Early-Career Scientists (22K14544), the Institute for Fermentation, Osaka (IFO) (IFO research grant Y-2022–2-012) and the Tsukuba Office for Meeting Opportunities (TOMO) Startup Sprout Support Program (Research Support Fund for Social Implementation) (grants to Masato Takahashi). This work was also supported in part by the JSPS KAKENHI Grant-in-Aid for Scientific Research B (22H02474) and Sumitomo Electric Industries Group Corporate Social Responsibility Foundation (2023–2028) (grants to Hideki Aoyagi).
Japan Society for the Promotion of Science, KAKENHI Grant-in-Aid for Early-Career Scientists (22K14544), JSPS KAKENHI Grant-in-Aid for Scientific Research B (22H02474), Institute for Fermentation, Osaka, IFO research grant Y-2022-2-012, Tsukuba Office for Meeting Opportunities (TOMO), Startup Sprout Support Program (Research Support Fund for Social Implementation), Sumitomo Electric Industries Group Corporate Social Responsibility Foundation, 2023-2028.
Author information
Authors and Affiliations
Contributions
Masato Takahashi and Yoshisuke Sawada designed the study. Yoshisuke Sawada and Masato Takahashi developed the culture system. Masato Takahashi and Hideki Aoyagi supervised the research. Masato Takahashi designed and performed all the experiments and analyzed the data. All authors wrote the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, M., Sawada, Y. & Aoyagi, H. A forced aeration system for microbial culture of multiple shaken vessels suppresses volatilization. Arch Microbiol 206, 246 (2024). https://doi.org/10.1007/s00203-024-03960-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-03960-2